Abstract
Background:
There has been an increased use of novel agents in the induction therapy for transplant-eligible AL amyloidosis over past decade. Hematologic response after an autologous hematopoietic stem cell transplantation (ASCT) is predictive of better outcomes, including organ response and overall survival. However, limited data exist about the outcomes of patients who are refractory to induction chemotherapy but proceed with upfront ASCT). We present here the outcomes of AL amyloidosis refractory to induction therapy.
Methods:
This retrospective study included all consecutive AL patients who had their ASCT at our institution between 01/2008 and 12/2018 and received induction therapy. We excluded patients who were untreated at the time of transplant. Primary objective: assess the hematologic response, progression-free survival (PFS) and overall survival (OS). Secondary objective: compare PFS and OS of AL amyloidosis by response to induction therapy (refractory vs sensitive). Refractory disease was defined as patient who had stable disease (SD) or progressive disease (PD) after at least 1 line of induction therapy. Hematologic response was defined per the 2012 consensus criteria. Survival estimates were calculated using Kaplan-Meier method.
Results:
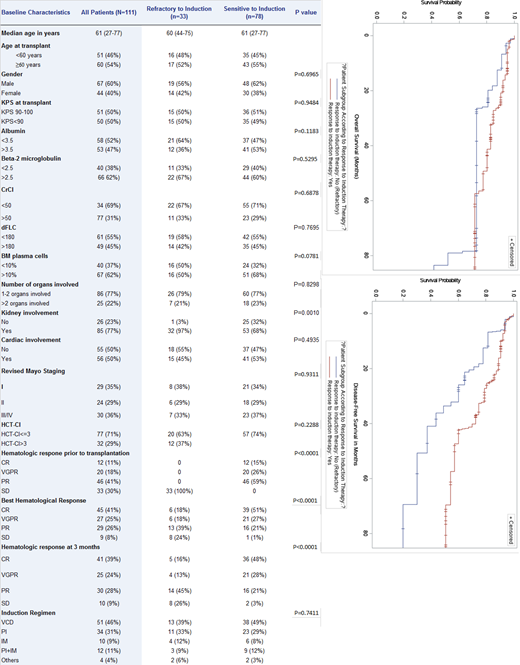
One-hundred-and-eleven patients with a median age of 61 (range, 27-77) years met eligibility criteria. Thirty-three (30%) were refractory and 78 (70%) were sensitive to induction therapy. Table 1 summarizes patient and disease characteristics of all study patients and for the refractory vs sensitive groups. Overall, the two groups were comparable except for significantly more kidney involvement in the refractory group (97% of patients). Induction therapies were similar in the two groups, with bortezomib/cyclophosphamide/dexamethasone (VCD) being the most commonly used regimen (46%). With a median follow-up of 3.11 (range, 0.18-11.15) years, the 3-year PFS and OS for all study patients were 67% and 78%, respectively. At 3 months after transplant, 74% of the patients in the refractory group achieved an objective hematologic response (OHR; defined as PR or better). Of these, 29% achieved VGPR/CR and 45% achieved PR. As expected, more patients in the sensitive group achieved OHR (97%) and VGPR/CR (76%). The respective 3-year PFS and OS were 49% and 73% in the refractory group compared to 75% and 83% in the sensitive group (p=0.0068 for PFS; p=0.0790 for OS). Univariate analysis (UVA) was performed for the variables listed in Table 1 and multivariable analyses included only factors with p value<0.1 in in the UVA. In MVA, in addition to increased risk for refractory patients (HR 2.885, 95% CI:1.237-6.729; p=0.0142), only elevated beta-2 microglobulin (HR 3.899, 95% CI:1.039-14.629; p=0.0437) was associated with inferior PFS. Regarding OS, age ≥60 (HR 3.812, 95% CI:1.038-14.002; p=0.0438) and revised Mayo stage III/IV (HR 3.886, 95% CI: 1.029-14.679; p=0.0453) were associated with inferior survival. In a subgroup analysis comparing PFS and OS stratifying patients by their response to induction (refractory vs sensitive) and their 3-month hematologic response after transplant, we found no significant differences in the 3-year PFS (86% for refractory vs 80% for sensitive group; p=0.7284) for those with VGPR or better but significantly inferior PFS for refractory patients who achieved <VGPR (27% compared to 74% for the sensitive group; p=0.0196).
Conclusion:
AL amyloid patients refractory to induction therapy seem to benefit from high-dose chemotherapy and ASCT in terms of both response rates and survival. Durable responses for refractory disease are notable in patients who achieved >VGPR after ASCT. Prospective studies comparing transplant versus non-transplant approaches are warranted for these high-risk patients.
Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Popat: Bayer: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Lee: Bristol Myers Squibb: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Janssen: Consultancy, Research Funding; Karyopharm: Consultancy; Legend Biotech: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Oncopetides: Consultancy; Takeda Pharmaceuticals: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Regeneron: Research Funding. Orlowski: Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria. Qazilbash: Janssen: Research Funding; Biolline: Research Funding; Angiocrine: Research Funding; Amgen: Research Funding; NexImmune: Research Funding; Bristol-Myers Squibb: Other: Advisory Board; Oncopeptides: Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal