Abstract
Survival of multiple myeloma (MM) has significantly improved over the past decade; however, a composed group of patients (15% to 20%), named high-risk (HR) MM, still experiences reduced survival. Both tumor biology and suboptimal/absent responses to therapy may underlie HR definition and a clear uniform identification of risk factors is crucial for proper management of these patients. In biologic HRMM, MRD attaining and sustaining negativity, inside and outside bone marrow, should be the primary goal and therapy should be adapted in patients with frailty to reduce toxicity and improve quality of life. MM treatment has traditionally been tailored to age and more recently frailty or comorbidities, but very rarely to the biology of the disease, mainly because of the lack of a clear benefit derived from a specific drug/combination, inhomogeneity in HR definition, and lack of data coming from prospective, properly designed clinical trials. Some attempts have been successfully made in this direction. In this review, we discuss the current definitions of HR and the need for a consensus, the results of available trials in HR patients, and the way through risk-adapted treatment strategies. For this purpose, we propose several clinical cases of difficult-to-treat patients throughout different treatment phases.
Introduction
The overall survival (OS) of patients with multiple myeloma (MM) has significantly improved over the past decade and is currently close to a median of 10 years for newly diagnosed (ND) fit patients.1,2 However, the improvement has not been uniform, and 15% to 20% of all patients have a predicted OS of less than 3 years. This subgroup is identified as having high-risk (HR) MM, and represents a challenge to diagnosis and to treat because of unsatisfactory disease control and early relapse, even with the newest therapies.3,4
The heterogeneity in clinical behavior of MM is influenced by many host- and disease-related factors, but the depth of response to therapy and resolution of imaging findings (referred to as dynamic factors) are also prognosticators. Patients with co-occurrence of HR features, particularly cytogenetic/genomic abnormalities, and/or early relapse (<1 year) after autologous stem cell transplantation (ASCT) are included into an ultra-HR category, with an expected median OS of less than 2 years.4,5 For these patients, innovative treatment strategies are warranted.
Therapeutic approaches to MM have traditionally been driven by patient’s age, frailty, comorbidities, and/or disease-related organ impairment. However, clinical trials mostly experiment the same strategies, whatever the risk imparted by the biology of the disease.6,7 Lacking prospective, risk-adapted studies, available data on the outcomes of HR patients are mostly biased by the post hoc nature of the analyses and reduced statistical power from the limited sample size of HR subgroups.
However, with the increased availability of highly active classes of novel drugs and innovative treatment strategies, the time has come to design prospective risk-tailored studies8-10 aimed at offering HR patients the most effective approaches to get and sustain minimal residual disease (MRD) negativity.11 In this review, we discuss the definitions of HRMM and the need for a consensus, results from available trials in HR patients, and the way through risk-adapted treatment strategies. We use several clinical cases of difficult-to-treat patients, each focusing on different risk aspects that might be applicable throughout different treatment phases.
Clinical case 1: treatment of ND HR ASCT-eligible MM
A 62-year-old woman was referred to us because of moderate anemia and a suspected serum M protein. She underwent a workup that established the diagnosis of active immunoglobulin A/K MM, International Staging System (ISS) stage III, with HR characteristics because of histologically proven involvement of hypermetabolic lymph nodes at positron emission tomography/computed tomography (PET/CT), 3% of circulating clonal PCs by flow cytometry, and positivity for del(13q) at conventional karyotype combined with amp(1q21) at fluorescent in situ hybridization (FISH) analysis.
MM is characterized by particularly heterogeneous clinical outcomes because of the high number of prognostic parameters related to the tumor load and to intrinsic cellular features. Additionally, patient-related factors often coexist, presenting a mixed scenario (Table 1).4,12,13
Prognostic factors in MM and cytogenetic abnormalities and relationship with outcomes
| Prognostic factors . | |||
|---|---|---|---|
| Patient-related . | Disease burden-related . | Disease biology-related . | Therapy-related . |
| Age | High B2 microglobulin* | Cytogenetic abnormalities | Quality of response |
| Performance status | Low albumin* | GEP | Early relapse |
| Comorbidities | Renal impairment | Circulating PCs | |
| LDH above ULN | EMD | ||
| High proliferation rate | |||
| Cytogenetic abnormalities and relationship with outcomes | |||
| Chromosome/region (frequency) | Gene involved/effect | Prognostic implication | |
| 14q32 (locus IGH) (45-50%) | |||
| t(11;14) (20%) | Cyclin D1 hyperexpression | Neutral | |
| t(4;14) (10% to 15%) | FGFR3 and MMSET deregulated | Unfavorable (worsened by chromosome 1 alterations, improved by trisomy 5) | |
| t(14;16) (<5%) | cMAF | Doubt, mainly unfavorable | |
| t(14;20) (<5%) | UK | Doubt, mainly unfavorable | |
| 1q21 acquisition (30%) | CKS1B, MCL1 | ||
| Gain (2-3 copies) | Partially unfavorable | ||
| Amplification (≥4) | Unfavorable | ||
| 1p32 deletion (10%) | FAF1/ CDKN2C | Unfavorable | |
| 17p deletion (8% to 15% according to PC cutoff) | TP53 and UK | ||
| Single-hit | Deletion | Unfavorable | |
| Double-hit | Biallelic inactivation (deletion + mutation) | Very unfavorable | |
| Prognostic factors . | |||
|---|---|---|---|
| Patient-related . | Disease burden-related . | Disease biology-related . | Therapy-related . |
| Age | High B2 microglobulin* | Cytogenetic abnormalities | Quality of response |
| Performance status | Low albumin* | GEP | Early relapse |
| Comorbidities | Renal impairment | Circulating PCs | |
| LDH above ULN | EMD | ||
| High proliferation rate | |||
| Cytogenetic abnormalities and relationship with outcomes | |||
| Chromosome/region (frequency) | Gene involved/effect | Prognostic implication | |
| 14q32 (locus IGH) (45-50%) | |||
| t(11;14) (20%) | Cyclin D1 hyperexpression | Neutral | |
| t(4;14) (10% to 15%) | FGFR3 and MMSET deregulated | Unfavorable (worsened by chromosome 1 alterations, improved by trisomy 5) | |
| t(14;16) (<5%) | cMAF | Doubt, mainly unfavorable | |
| t(14;20) (<5%) | UK | Doubt, mainly unfavorable | |
| 1q21 acquisition (30%) | CKS1B, MCL1 | ||
| Gain (2-3 copies) | Partially unfavorable | ||
| Amplification (≥4) | Unfavorable | ||
| 1p32 deletion (10%) | FAF1/ CDKN2C | Unfavorable | |
| 17p deletion (8% to 15% according to PC cutoff) | TP53 and UK | ||
| Single-hit | Deletion | Unfavorable | |
| Double-hit | Biallelic inactivation (deletion + mutation) | Very unfavorable | |
UK, unknown; ULN, upper limit of normal.
ISS.
Risk factors in MM (Table 1) may reflect both the tumor burden and the intrinsic biology of neoplastic cells.4 Among all prognostic factors, genetic-molecular alterations and response to treatment are the most robust predictors of outcomes.
The first and most widely available prognostic system in MM is the ISS, based on beta 2-microglobulin (beta2-mic) and albumin14 levels, which outperformed the original Durie-Salmon staging system,15 stratifying patients into 3 groups. The major limitations of the ISS are that the patients studied to define the criteria were treated with old combinations, which were not representative of current standard of care, and the lack of inclusion of genomic proliferation-related aspects. To face the latter limitation, a revised ISS (R-ISS), has recently been proposed,16 as discussed later.
Extramedullary disease (EMD) and/or plasma cell leukemia (PCL) are HR plasma cell (PC) neoplasms characterized by the spread of PCs outside the bone marrow (BM). EMD arises in soft tissues, completely disconnected from the BM/bones, and should be differentiated from paraskeletal or paramedullary disease, consisting of soft-tissue masses arising from bone lesions and displaying a better prognosis.17 Whenever possible, a biopsy of the tissue is desirable. Central nervous system involvement is associated with the worst clinical outcomes.17 Although PCL has traditionally been defined by more than 2 × 109 (or 20%) circulating PCs,18 more recent studies have reported unfavorable outcomes also for patients carrying lower numbers,19 supporting the adverse impact of circulating PCs.
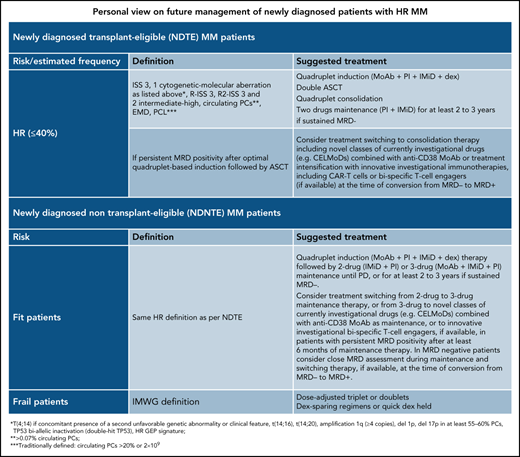
To facilitate the reader having a concise picture, our personal view on risk classification of newly diagnosed patients is presented in Table 2.
Personal view on future management of newly diagnosed patients with HR MM
| Newly diagnosed transplant-eligible (NDTE) patients with MM . | ||
|---|---|---|
| Risk (estimated frequency) . | Definition . | Suggested treatment . |
| HR (≤40%) | ISS 3, 1 cytogenetic-molecular aberration as listed*, R-ISS 3, R2-ISS 3 and 2 intermediate-high, circulating PCs†, EMD, PCL‡ | Quadruplet induction (MoAb + PI + IMiD + dex) Double ASCT Quadruplet consolidation Two-drug maintenance (PI + IMiD) for ≥2-3 y if sustained MRD− |
| If persistent MRD positivity after optimal quadruplet-based induction followed by ASCT | Consider switching treatment to consolidation therapy including novel classes of currently investigational drugs (eg, CELMoDs) combined with anti-CD38 MoAb or treatment intensification with innovative investigational immunotherapies, including CAR T cells or bispecific T-cell engagers (if available) at the time of conversion from MRD− to MRD+ | |
| Newly diagnosed non-transplant-eligible (NDNTE) patients with MM | ||
| Fit patients | Same HR definition as per NDTE | Quadruplet induction (MoAb + PI + IMiD + dex) therapy followed by 2-drug (IMiD + PI) or 3-drug (MoAb + IMiD + PI) maintenance until PD, or for at least 2-3 y if sustained MRD−. Consider switching treatment from 2-drug to 3-drug maintenance therapy, or from 3-drug to novel classes of currently investigational drugs (eg, CELMoDs) combined with anti-CD38 MoAb as maintenance, or to innovative investigational bispecific T-cell engagers, if available, in patients with persistent MRD+ after at least 6 mo of maintenance therapy. In MRD− patients, consider close MRD assessment during maintenance and switching therapy, if available, at the time of conversion from MRD− to MRD+. |
| Frail patients | IMWG definition | Dose-adjusted triplet or doublets Dex-sparing regimens or quick dex held |
| Newly diagnosed transplant-eligible (NDTE) patients with MM . | ||
|---|---|---|
| Risk (estimated frequency) . | Definition . | Suggested treatment . |
| HR (≤40%) | ISS 3, 1 cytogenetic-molecular aberration as listed*, R-ISS 3, R2-ISS 3 and 2 intermediate-high, circulating PCs†, EMD, PCL‡ | Quadruplet induction (MoAb + PI + IMiD + dex) Double ASCT Quadruplet consolidation Two-drug maintenance (PI + IMiD) for ≥2-3 y if sustained MRD− |
| If persistent MRD positivity after optimal quadruplet-based induction followed by ASCT | Consider switching treatment to consolidation therapy including novel classes of currently investigational drugs (eg, CELMoDs) combined with anti-CD38 MoAb or treatment intensification with innovative investigational immunotherapies, including CAR T cells or bispecific T-cell engagers (if available) at the time of conversion from MRD− to MRD+ | |
| Newly diagnosed non-transplant-eligible (NDNTE) patients with MM | ||
| Fit patients | Same HR definition as per NDTE | Quadruplet induction (MoAb + PI + IMiD + dex) therapy followed by 2-drug (IMiD + PI) or 3-drug (MoAb + IMiD + PI) maintenance until PD, or for at least 2-3 y if sustained MRD−. Consider switching treatment from 2-drug to 3-drug maintenance therapy, or from 3-drug to novel classes of currently investigational drugs (eg, CELMoDs) combined with anti-CD38 MoAb as maintenance, or to innovative investigational bispecific T-cell engagers, if available, in patients with persistent MRD+ after at least 6 mo of maintenance therapy. In MRD− patients, consider close MRD assessment during maintenance and switching therapy, if available, at the time of conversion from MRD− to MRD+. |
| Frail patients | IMWG definition | Dose-adjusted triplet or doublets Dex-sparing regimens or quick dex held |
CELMoDs, cereblon E3 ligase modulator; dex, dexamethasone; HR, high-risk; PD, progressive disease.
t(4;14) if concomitant presence of a second unfavorable genetic abnormality or clinical feature, t(14;16), t(14;20), amplification 1q (≥4 copies), del1p, del17p in at least 55% to 60% PCs, TP53 biallelic inactivation (double-hit TP53), HR GEP signature.
>0.07% circulating PCs.
Traditionally defined: circulating PCs >20% or 2 × 109.
Cytogenetic-molecular aberrations and staging systems
FISH analysis of CD-138+ BM PCs is the preferred and routinely used method to detect recurrent chromosomal abnormalities.4,20 FISH testing can reveal abnormalities in ∼90% of patients, but only provides information on the regions interrogated by probes.
The main chromosomes and genes involved, their frequency, and prognostic implications are described in Table 1. Overall, hyperdiploidy (present in 55% of cases) and translocations (t) involving the immunoglobulin heavy chain (IGH) locus at the chromosome 14q32 region (45% to 50% of cases) are the most common abnormalities and may impact prognosis differently. Among these, trisomies (55% of cases), preferentially affecting the odd-numbered chromosomes, and t(11;14) (20%) resulting in cyclin D1 hyperexpression, are mainly grouped as standard risk alterations.21 t(4;14) deregulating the F GFR3 and MMSET genes is the second most frequent translocation (10% to 15%), whereas others, in particular t(14;16) involving the cMAF gene and t(14;20)3,20 are less represented (5%). Although t(4;14) has been associated with a poor outcome in several studies,22 its prognostic role may be either worsened or mitigated by the presence of cosegregating abnormalities, such as del(1p32) and trisomy 5, respectively, and/or other clinical features. In several studies, bortezomib-based treatments have improved, or even overcome, the poor prognosis imparted by t(4;14).23-25
Chromosome 1q21 abnormalities, including gain of the long arm (1q+, defined as only 1 additional copy of 1q, or a total of 3 copies) and amplification of chromosome 1 [amp(1q), defined as ≥2 extra copies, or ≥4 total copies of 1q], lead to dysregulation of several genes (CKS1B, MCL1, or others26), and occur in 40% of ND MM. Both these abnormalities are at HR, amp(1q) being probably more detrimental on outcome.27,28 Also, the deletion of the short arm of chromosome 1, 1p32, targeting FAF1 and CDKN2C genes, present in ∼10% of the patients, is associated with poor prognosis.29
Finally, deletion of the short arm of chromosome 17, del(17p), and/or monosomy 17 are undoubtfully the worst genetic prognostic factors,30,31 though still associated with some grade of uncertainty.32 These latter include the size of the PC clone with del(17p),31-33 the highest prognostic significance being recently suggested to be set at 55% to 60%,34 and the molecular target of del17p, with the TP53 gene being involved only in some cases, and the worst prognosis being associated with the biallelic inactivation of TP53 because of deletion plus mutation (referred to as “double-hit” TP53 MM).35-37
Genetic abnormalities captured by FISH cannot account for the huge molecular heterogeneity of MM, leading to the evaluation of other approaches, such as gene expression profiling (GEP) and next-generation sequencing (NGS).4,38 Several GEP signatures have been developed to define risk, including different gene numbers,39-42 with only a partial overlap, and subsequently difficult to compare one with the other. For this reason, these signatures never entered in routine clinical practice. Also, single nucleotide polymorphism-based microarrays are valid tools in detecting copy number abnormalities and may be used in place of FISH, but they cannot identify translocations, inversions, or point mutations.43 The most frequently mutated genes are KRAS and NRAS (20% to 25% patients), followed by BRAF and FAM46C (10%) and TP53 (5% to 6%); the prognostic value of these mutations is often dependent on the burden and signature.44,45
In 2016, the International Myeloma Working Group (IMWG) made an attempt to find a consensus for the definition of HR cytogenetics MM,3 identifying patients with t(4;14), t(14;16), t(14;20), del17p, gain 1q, nonhyperdiploid karyotype, del13q at conventional karyotype, and HR signature at GEP as HR and all the others being standard risk. Also, a revision of the ISS with the inclusion of 3 genomic alterations [t(4;14), t(14;16) and del(17p)] and lactate dehydrogenase (LDH) values have been proposed (R-ISS), stratifying patients in 3 stages, with significant distinct OS.16,46 An integrate analysis of clinical and whole-genome and exome data on 784 patients identified a HR group of patients (6% of the population of ND MM), named double-hit, characterized by either a biallelic TP53 inactivation or 1q amplification (≥4 copies) in addition to ISS stage 3, with a dismal prognosis.47 Finally, a new scoring system, tested on >2000 ND MM patients enrolled into different prospective clinical trials, adding to R-ISS chromosome 1q gain/amplification (so-called R2-ISS), proved to split patients into 4 different risk categories, better differentiating the R-ISS II stage, quite heterogeneous, into 2 groups, the intermediate-low risk and the intermediate-high risk.48
Despite these attempts providing reliable prognostic tools for risk assessment, they are far from being comprehensive, up-to-date on newer identified molecular lesions and are oversimplified; for example, not considering co-occurrence of genetic abnormalities or of different risk factors, or that therapy may affect the importance of each one. The Intergroupe Francophone du Myelome recently proposed a relatively simple prognostic score based on FISH and able to outperform the R-ISS, including 5 abnormalities with unfavorable meaning (trisomy 21, t(4;14), gain 1q, del1p32, del 17p) and 1 with protective value (trisomy 5) to split patients into 3 risk categories.34 Of course, other scores may be applied, including NGS, to allow incorporating the assessment of IGH translocations, recurrent mutations, and copy number variations43 at once.
Back to the clinical case
The patient was classified as HR for the presence of cytogenetic aberrations, ISS 3, circulating PCs, and EMD and treated according to national guidelines with 4 cycles of bortezomib-thalidomide-dexamethasone (VTD) induction, achieving stringent complete response (sCR), followed by double ASCT (conditioned with 200 mg/m2 melphalan), and thereafter 2 cycles of VTD consolidation. After the consolidation phase, the patient maintained sCR and assessment of MRD status by NGS (sensitivity level of 10−5) was negative. Maintenance therapy with lenalidomide (len) was started following consolidation and is ongoing after 18 months from diagnosis.
Best available options for first-line treatment
The optimal choice of novel agent combinations and their most effective incorporation into the treatment paradigm for patients with HRMM who are candidates to receive ASCT remain not well defined (Table 3). These uncertainties rely on the lack of homogeneous criteria for defining HR disease and of available data from clinical trials designed to prospectively compare standard-of-care therapies vs intensified treatment strategies in the HR setting. As a result, guidelines from European, US, and international cooperative groups suggest variable therapeutic approaches to patients with MM who are at HR. Notably, essential endpoints of treatment interventions for these patients are to maximize the rate of undetectable MRD at a sensitivity level of at least 10−5 and to sustain MRD negativity with longer term drug exposure than usually planned in patients at standard risk. According to the IMWG, the optimal induction therapy for patients with HR cytogenetics should include a proteasome inhibitor (PI), such as bortezomib, plus an immunomodulatory drug (IMiD), preferentially len, and dexamethasone (VRd),3,24 with a number of cycles variable between 4 and 6, according to the maximal response, and is recommended also in IMWG,49 European Myeloma Network (EMN),50 and American Society of Clinical Oncology/Cancer Care Ontario guidelines,51 regardless of the risk status. Results from several phase 2 studies demonstrating the efficacy of carfilzomib plus lenalidomide and dexamethasone (KRd) combined or not with ASCT in inducing substantially high rates of complete response (CR) and deepening the response below the level of detectable MRD have supported the inclusion of KRd in the latest National Comprehensive Cancer Network (NCCN) guidelines.52 VTd, VRd, or KRd have more recently represented the backbone to which add daratumumab (Dara) or isatuximab with the objective to further increase the activity.53-59 Results from these studies reported unprecedented high rates of MRD negativity, up to 70% and even in patients with HR disease, and promising progression-free survival (PFS), though in some of them the follow-up was still short. Building on these promising data,60,61 large clinical trials have been designed to definitely support the preferential use of these quadruplets in patients at HR. The addition of polychemotherapy to triplet induction regimens, even though not supported by phase 3 trials, may be considered in patients with EMD and PCL. The use of double ASCT in ND MM was introduced before novel agents’ availability and depended on raising the dose intensity of melphalan. More recently, several studies have readdressed its role for patients with ND MM, particularly those at HR. Results from a retrospective pooled analysis of 3 phase 3 studies supporting the superior outcomes with double over single ASCT for patients with advanced ISS clinical stage and HR cytogenetics58 were recently confirmed and extended by the final analysis of the EMN02 study prospectively comparing single with double ASCT.62 Although double ASCT significantly prolonged PFS and OS in the intention-to-treat population, the greatest reduction in the risk of progression or death was observed in patients with HR cytogenetics, particularly those with del(17p). These results were not confirmed in the primary analysis of the US StaMINA study,63 though the 2 trials cannot be easily compared because of major differences in their design, including the preferred use of longer VRd induction (median, 5 cycles) in StaMINA vs shorter Vd plus cyclophosphamide (VCd) induction (median, 4 cycles) in EMN02. To overcome the potential dilution of double ASCT effect because of the high nonadherence rate (32%) to this randomly planned treatment in the StaMINA study, a per-protocol analysis was recently performed.63,64 Overall, the estimated 6-year PFS rate for patients in the double ASCT group was significantly longer compared with the other groups, and this benefit was clinically relevant in patients at HR. Although the debate is still open, double ASCT is recommended for patients at HR in IMWG and EMN guidelines6,50 and is considered a treatment option for the same subgroup of patients in NCCN guidelines52 and in the Mayo stratification for myeloma and risk-adapted treatment guidelines.12 A balanced interpretation of results from these studies is further complicated by the apparent lack of impact on PFS by the length of induction therapy (<4 vs >4 months),58,63 as well as of similar PFS curves for patients with del(17p) positivity who received VRd or VCd induction therapy before ASCT.65
Results of selected prospective clinical trials for newly diagnosed-transplant eligible patients carrying high-risk features
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | No. HR patients (%) . | Outcomes in HR vs SR . | PFS rates . | MRD− (%) . |
|---|---|---|---|---|---|---|---|
| CASSIOPEA53,115 | Dara-VTd vs VTd | Phase 3, transplant eligible (sCR at 100 d post-ASCT) | del(17p) ≥50% or t(4;14) ≥30% | 168 (15.5) | Prespecified subgroup analysis (sCR) showed consistent treatment benefit of D-VTd over VTd except for HR pts. However, ≥CR rates in HR pts favored D-VTd vs VTd (36.6% vs 32.6%; OR, 1.11; 95% CI, 0.58- 2.10). | D-VTd vs VTd reduced risk of progression/death (-53%) (median FU 18.8 mo): HzR 0.67; 95% CI, 0.35-1.30 (HR group) HzR 0.47; 95% CI, 0.33-0.67 (SR group) | 10−5 MRD post-cons (D-VTd vs VTd): 59.8% vs 44.2% (HR pts; OR, 1.88; 95% CI, 1.02-3.46) 63.7% vs 43.5% (all pts; OR, 2.27; 95% CI, 1.78-2.90; P < .0001) |
| GRIFFIN54 | Dara-VRd vs VRd | Phase 2, transplant eligible (sCR at the end of post-ASCT cons) | t(4;14), t(14;16), or del(17p) | 30 (15.4) | Subgroup analysis of sCR (end of post-ASCT at 13.5 mo): 18.8% (D-RVd) vs 30.8% (RVd), (OR, 0.52 95% CI, 0.09-2.90) | Median PFS not reached in either group. Insufficient power to analyze HR subgroup of pts | 10−5 MRD− at 22.1 mo FU (D-RVd vs RVd): 37.5 vs 28.6 (HR) 54.9 vs 20.5 (SR) 51.0 vs 20.4 (ITT) 47.1 vs 18.4 (ITT ≥CR) |
| STAMINA63,116 | ASCT + len maintenance (auto/len) vs ASCT + VRd consolidation + len maintenance (auto/VRd) vs tandem ASCT + len maintenance (auto/auto) | Phase 3, transplant eligible (38 mo PFS) | β2M > 5.5 mg/L, t(4;14), t(14;20), t(14;16), del (17p), del(13) detected by SC only, or aneuploidy | 223 (29) | 38-mo estimates PFS (95% CI): 57.6% (auto/len) vs 61.6% (auto/VRd) vs 62.9% (auto/auto) P value unavailable 6-y PFS in HR pts as treated analysis were 43.6% and 26% for auto/auto and auto/len, respectively (P = 0.03). | 38-mo estimates (95% CI): 57.6% (HR) vs 53.9% (SR) (auto/len) 61.6% (HR) vs 57.8% (SR) (auto/VRd) 62.9% (HR) vs 58.5% (SR) (auto/auto) PFS at 6 y (ITT population, P = 0.6): 40.9% (auto/len), 39.7% (auto/VRD), 43.9 (auto/auto), PFS at 6 y (as treated population, P = 0.03): 26% (HR) vs 38.6% (SR) (auto/len) NR (HR) vs 39.7% (SR) (auto/VRd) 43.6% (HR) vs 49.4% (SR) (auto/auto) | — |
| EMN02/HO9562 | VCD, followed by VMp or ASCT (single or tandem) | Phase 3, transplant eligible (PFS) | t(4;14) ≥10%, t(14;16) ≥10%, or del(17p) ≥20% | 225 (19) | Median PFS: 20.3 mo (VCD/VMp) vs 37.3 mo (VCD/ASCT), HzR 0.63 (95% CI, 0.46-0.88) | Median PFS: 20.3 mo (HR) vs 46.7 mo (SR) (VMp) 37.3 mo (HR) vs NR (SR) (ASCT) | — |
| GMMG-HD6 (NCT02495922) | VRD ± elo in induction and consolidation, followed by len-dex ± elo maintenance | Phase 3, transplant eligible (PFS) | — | — | Ongoing study | — | — |
| EMN18 (NCT03896737) | Dara-VCd vs VTd and ASCT, followed by Ixa ± Dara maintenance | Phase 3, transplant eligible (PFS, MRD neg) | del(17p) (≥ 10) or t(4;14) (≥ 15) or t(14;16) (≥ 15) | — | Ongoing study | — | — |
| GEM14 (NCT02253316) | Ongoing study | Phase II, transplant eligible (PFS) | — | — | Ongoing study | — | — |
| PERSEUS (NCT03710603) | VRd ± Dara followed by Len ± Dara maintenance | Phase III, transplant eligible (PFS) | del17p, t[4;14] and t[14;16], amp(1q21) | — | Ongoing study | — | — |
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | No. HR patients (%) . | Outcomes in HR vs SR . | PFS rates . | MRD− (%) . |
|---|---|---|---|---|---|---|---|
| CASSIOPEA53,115 | Dara-VTd vs VTd | Phase 3, transplant eligible (sCR at 100 d post-ASCT) | del(17p) ≥50% or t(4;14) ≥30% | 168 (15.5) | Prespecified subgroup analysis (sCR) showed consistent treatment benefit of D-VTd over VTd except for HR pts. However, ≥CR rates in HR pts favored D-VTd vs VTd (36.6% vs 32.6%; OR, 1.11; 95% CI, 0.58- 2.10). | D-VTd vs VTd reduced risk of progression/death (-53%) (median FU 18.8 mo): HzR 0.67; 95% CI, 0.35-1.30 (HR group) HzR 0.47; 95% CI, 0.33-0.67 (SR group) | 10−5 MRD post-cons (D-VTd vs VTd): 59.8% vs 44.2% (HR pts; OR, 1.88; 95% CI, 1.02-3.46) 63.7% vs 43.5% (all pts; OR, 2.27; 95% CI, 1.78-2.90; P < .0001) |
| GRIFFIN54 | Dara-VRd vs VRd | Phase 2, transplant eligible (sCR at the end of post-ASCT cons) | t(4;14), t(14;16), or del(17p) | 30 (15.4) | Subgroup analysis of sCR (end of post-ASCT at 13.5 mo): 18.8% (D-RVd) vs 30.8% (RVd), (OR, 0.52 95% CI, 0.09-2.90) | Median PFS not reached in either group. Insufficient power to analyze HR subgroup of pts | 10−5 MRD− at 22.1 mo FU (D-RVd vs RVd): 37.5 vs 28.6 (HR) 54.9 vs 20.5 (SR) 51.0 vs 20.4 (ITT) 47.1 vs 18.4 (ITT ≥CR) |
| STAMINA63,116 | ASCT + len maintenance (auto/len) vs ASCT + VRd consolidation + len maintenance (auto/VRd) vs tandem ASCT + len maintenance (auto/auto) | Phase 3, transplant eligible (38 mo PFS) | β2M > 5.5 mg/L, t(4;14), t(14;20), t(14;16), del (17p), del(13) detected by SC only, or aneuploidy | 223 (29) | 38-mo estimates PFS (95% CI): 57.6% (auto/len) vs 61.6% (auto/VRd) vs 62.9% (auto/auto) P value unavailable 6-y PFS in HR pts as treated analysis were 43.6% and 26% for auto/auto and auto/len, respectively (P = 0.03). | 38-mo estimates (95% CI): 57.6% (HR) vs 53.9% (SR) (auto/len) 61.6% (HR) vs 57.8% (SR) (auto/VRd) 62.9% (HR) vs 58.5% (SR) (auto/auto) PFS at 6 y (ITT population, P = 0.6): 40.9% (auto/len), 39.7% (auto/VRD), 43.9 (auto/auto), PFS at 6 y (as treated population, P = 0.03): 26% (HR) vs 38.6% (SR) (auto/len) NR (HR) vs 39.7% (SR) (auto/VRd) 43.6% (HR) vs 49.4% (SR) (auto/auto) | — |
| EMN02/HO9562 | VCD, followed by VMp or ASCT (single or tandem) | Phase 3, transplant eligible (PFS) | t(4;14) ≥10%, t(14;16) ≥10%, or del(17p) ≥20% | 225 (19) | Median PFS: 20.3 mo (VCD/VMp) vs 37.3 mo (VCD/ASCT), HzR 0.63 (95% CI, 0.46-0.88) | Median PFS: 20.3 mo (HR) vs 46.7 mo (SR) (VMp) 37.3 mo (HR) vs NR (SR) (ASCT) | — |
| GMMG-HD6 (NCT02495922) | VRD ± elo in induction and consolidation, followed by len-dex ± elo maintenance | Phase 3, transplant eligible (PFS) | — | — | Ongoing study | — | — |
| EMN18 (NCT03896737) | Dara-VCd vs VTd and ASCT, followed by Ixa ± Dara maintenance | Phase 3, transplant eligible (PFS, MRD neg) | del(17p) (≥ 10) or t(4;14) (≥ 15) or t(14;16) (≥ 15) | — | Ongoing study | — | — |
| GEM14 (NCT02253316) | Ongoing study | Phase II, transplant eligible (PFS) | — | — | Ongoing study | — | — |
| PERSEUS (NCT03710603) | VRd ± Dara followed by Len ± Dara maintenance | Phase III, transplant eligible (PFS) | del17p, t[4;14] and t[14;16], amp(1q21) | — | Ongoing study | — | — |
β2M, β2 microglobulin; cons, consolidation; CI, confidence interval; CR, complete response; FU, follow-up; HR, high-risk; HzR, hazard ratio; ITT, intention-to-treat population; Ixa, ixazomib; len, lenalidomide; NR, not reported; ns, not significant; OR, odds ratio; PFS, prgression-free survival; pts, patients; SC, standard cytogenetics; sCR, stringent complete response; SR, standard risk; VCd, bortezomib, cyclophosphamide and dexamethasone; VMp, bortezomib, melphalan, prednisone; VRd, bortezomib, lenalidomide, dexamethasone; VTd, bortezomib, thalidomide, dexamethasone.
Consolidation therapy typically includes the same combination of agents used as induction therapy before ASCT, and is given for a number of fixed cycles, more often in the range of 2 to 4.66 Many phase 2 and 3 studies comparing 3- vs 2-drug consolidation regimens, as well as quadruplet vs triplet therapies, have been performed so far, supporting the value of consolidation therapy in increasing the rate of high-quality responses, including stringent CR and MRD negativity.67 The role of consolidation therapy with VRd vs no consolidation was prospectively evaluated in 2 randomized studies, with conflicting findings. Indeed, positive results reported in the EMN02 study68 were not confirmed by the US StaMINA study,63 leading to heterogeneous recommendations in different guidelines. Whether the less or more relevant impact of post-ASCT consolidation therapy on subsequent outcomes may be influenced by the length of induction therapy received before ASCT is still debated. Assessment of MRD status at the highest sensitivity level achievable with modern techniques may help the physician to make clinical decision in the HR setting. For these patients, sequential assessment of MRD at different treatment phases is likely to represent the platform to build an MRD-driven therapy aimed at intensifying therapy (eg, switching to different classes of agents or modifying the treatment strategy, including performing double ASCT or proposing chimeric antigen receptor T-cell therapy [CAR-T] or T-cell engagers) in MRD+ patients. Studies aimed at establishing evidence to support these different approaches are still lacking, and should be provided.
Lenalidomide is the only novel agent approved for maintenance therapy after ASCT, and is the current standard of care based on improved PFS and OS reported in the overall patient population and in many subgroups of patients.69 Discordant findings related to the presence, or absence, of OS benefit with len in patients with HR cytogenetics69,70 led several groups to recommend in this setting maintenance therapy with a PI such as bortezomib, either as a single agent or combined with len, or even a PI- and IMiD-based triplet, like VRd.25,71-73 Again, there are few studies prospectively designed to deliver a risk-adapted maintenance approach based either on the risk at baseline or the risk at the time of starting maintenance (eg, persistent MRD positivity). In a retrospective study, risk-adapted VRd maintenance for up to 3 years improved both PFS and OS for HR patients who, however, had worst clinical outcomes than standard-risk patients receiving len alone.71 The role of ixazomib and daratumumab, each of them given either as a single agent or combined with another drug or with each other, as well as doublets like Dara-R or KR in the maintenance strategy after ASCT are under investigation.53,74-76 Moreover, studies are under way to prospectively define the optimal duration of maintenance therapy (eg, a treat-to-progression or fixed-duration approach), with an MRD-driven strategy.
Clinical case 2: treatment of NDHR ASCT-ineligible MM
A 78-year-old man with a history of prostatectomy for adenocarcinoma, atrial fibrillation in oral anticoagulant therapy, hypercholesterolemia, and type 2 diabetes was admitted to the hospital because of worsening lumbar pain and laboratory evidence of moderate renal failure (serum creatinine 2.6 mg/dL, estimated glomerular filtration rate 35 mL/min). The workup led to a final diagnosis of immunoglobulin G-K MM, R-ISS stage III from a beta2-micr level of 15 mg/L and presence of t(4;14) at FISH, and need for immediate therapy because of extensive skeletal disease documented by fluorodeoxyglucose PET/CT and axial magnetic resonance imaging.
Defining elderly patients’ fitness and risk score
More than one-half of patients with NDMM are considered elderly (≥65 years) and because this population is extremely heterogeneous, with comorbidities and frailty increasing with the increasing age, which per se confers a higher death risk.77 In addition, the presence of additional HR features may further worsen the outcomes.78 Geriatric assessments and frailty scores have been recommended for use in oncogeriatric practice to stratify patients’ vulnerability to adverse outcomes when exposed to specific stressors, such as cancer and its treatment, and to tailor drug regimens.79 Also, with age, the presence of comorbidities increases,80 the most common being diabetes mellitus, hypertension, heart failure, cardiac arrhythmias, hyperlipidemia, chronic renal failure, and other cancers. Drug discontinuation secondary to toxicity and grade 3-4 adverse events during treatment are also associated with reduced OS in patients with MM.81
Several risk scores, either simple or more complicated ones, have been proposed in MM, the most widely used being proposed by the IMWG,82 and a recent meta-analysis confirmed their validity in predicting death risk.79,83-85 It was recently demonstrated that age >80 years old confers superimposable frailty, as well as the other components of the IMWG score.82 Several limitations, including inter-observer variability in judgment, time-consuming assessments, biological vs chronological age, and the lack on comorbidity information within clinical trials, are present in these geriatric assessments, and efforts are ongoing to refine them.
Back to the clinical case
After an accurate evaluation of the age, frailty status of the patient (renal failure, hypomobility), and the presence of comorbidities, which categorized him as intermediate/unfit, and on the other side the intermediate cytogenetic risk, the patient started reduced-dose len-dexamethasone (dex) and daratumumab, allowing him, after the first 8 weeks, to receive most of the treatment at home. He achieved partial response after 2 cycles, very good partial response after 5 cycles, and CR at cycle 9. Renal function improved to an estimated glomerular filtration rate of 45 mL/min. The patient is currently receiving monthly Dara (XVI cycle), len 10 mg, and he stopped dex, in light of the optimal response, the underlying diabetes, and the COVID-19 pandemic.
Best available options for first-line treatment of ASCT-ineligible patients
Improvements in outcomes for elderly patients with HR features have not been evident as in transplant-eligible patients because it is difficult to combine the need to provide long-term benefits to survival, while keeping minimal toxicity (Table 4).78 Moreover, a relatively small number of patients with frailty characteristics are usually enrolled in the main prospective clinical trials, making this population underrepresented and statistical results less reliable.
Results of selected prospective clinical trials for newly diagnosed non-transplant-eligible patients carrying high-risk features
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | No. HR patients (%) . | Outcomes in HR vs SR . | PFS rates . | MRD− (%) . |
|---|---|---|---|---|---|---|---|
| SWOG-12118 | elo-VRd vs VRd | Phase 2, only HR patients, transplant ineligible (PSF) | HR-GEP, t(14;16), t(14;20), del (17p), amp(1q21), primary PCL, or elevated serum LDH (≥2 × ULN) | 100 (100) | Median FU 53 mo: no difference in median PFS | Median PFS: 31.47 mo (elo-VRd) vs 33.64 mo (VRd) P = .45 | — |
| SWOG S077786 | VRd vs Rd | Phase 3, transplant ineligible (PSF) | t(4;14), t(14;16), or del (17p) | 104 (33) | Median PFS in HR pts: 38 (VRd) vs 16 (Rd) mo*P = .19 34 (VRd) vs 17 (Rd) mo†P = .96 (median overall FU 55 mo) | Unstratified median PFS: 43 mo (VRd) vs 30 mo (Rd) | — |
| ALCYONE88,94 | D-VMp vs VMp | Phase 3, transplant ineligible (PSF) | t(4;14), t(14;16), or del(17p) | 98 (14) | PFS in HR pts NR HzR (95% CI) 0.78 (0.4-1.43) CR rate (HR) and MRD (HR) NR | HR pts: NR vs NR ITT: 36.4 mo (D-VMp) vs 19.3 mo (VMp) | HR pts: NR vs NR ITT: 28% (D-VMp) vs 7% (VMp) |
| MAIA87,92 | D-Rd vs Rd | Phase 3, transplant ineligible (PSF) | t(4;14), t(14;16), or del(17p) | 92 (12) | PFS in HR pts: NR (D-Rd) vs 29.6 mo (Rd) HzR: (95% CI) 0.57 (0.32-1.04) CR rate (HR) and MRD (HR) NR | HR pts: 45.3 (D-Rd) vs 29.6 mo (Rd) ITT: NR (D-Rd) vs 38.8 mo (Rd) | HR pts: NR vs NR ITT: 29% (D-Rd) vs 9% (Rd) |
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | No. HR patients (%) . | Outcomes in HR vs SR . | PFS rates . | MRD− (%) . |
|---|---|---|---|---|---|---|---|
| SWOG-12118 | elo-VRd vs VRd | Phase 2, only HR patients, transplant ineligible (PSF) | HR-GEP, t(14;16), t(14;20), del (17p), amp(1q21), primary PCL, or elevated serum LDH (≥2 × ULN) | 100 (100) | Median FU 53 mo: no difference in median PFS | Median PFS: 31.47 mo (elo-VRd) vs 33.64 mo (VRd) P = .45 | — |
| SWOG S077786 | VRd vs Rd | Phase 3, transplant ineligible (PSF) | t(4;14), t(14;16), or del (17p) | 104 (33) | Median PFS in HR pts: 38 (VRd) vs 16 (Rd) mo*P = .19 34 (VRd) vs 17 (Rd) mo†P = .96 (median overall FU 55 mo) | Unstratified median PFS: 43 mo (VRd) vs 30 mo (Rd) | — |
| ALCYONE88,94 | D-VMp vs VMp | Phase 3, transplant ineligible (PSF) | t(4;14), t(14;16), or del(17p) | 98 (14) | PFS in HR pts NR HzR (95% CI) 0.78 (0.4-1.43) CR rate (HR) and MRD (HR) NR | HR pts: NR vs NR ITT: 36.4 mo (D-VMp) vs 19.3 mo (VMp) | HR pts: NR vs NR ITT: 28% (D-VMp) vs 7% (VMp) |
| MAIA87,92 | D-Rd vs Rd | Phase 3, transplant ineligible (PSF) | t(4;14), t(14;16), or del(17p) | 92 (12) | PFS in HR pts: NR (D-Rd) vs 29.6 mo (Rd) HzR: (95% CI) 0.57 (0.32-1.04) CR rate (HR) and MRD (HR) NR | HR pts: 45.3 (D-Rd) vs 29.6 mo (Rd) ITT: NR (D-Rd) vs 38.8 mo (Rd) | HR pts: NR vs NR ITT: 29% (D-Rd) vs 9% (Rd) |
CI, confidence interval; CR, complete response; D-, daratumumab; elo, elotuzumab; GEP, gene expressing profiling; HR, high-risk; HzR, hazard ratio; ITT, intention-to-treat; LDH, lactate dehydrogenase; NE, not estimable; NR, not reported; NS, not significant; Rd, lenalidomide, dexamethasone; PCL, plasma cell leukemia; PFS, progression-free survival; pts, points; SR, standard risk; ULN, upper limit of normal; VMp, bortezomib, melphalan, prednisone; VRd, bortezomib, lenalidomide, dexamethasone.
In the 44 pts HR by FISH.
In the 17 pts with t(4;14) by FISH.
In fit patients, the combination of first-generation novel agents, PIs or IMiDs, have improved the response rate in the HR subset, although OS remained significantly shorter, probably also for the limited number of patients with available data.78,86 Even in the recent phase 3 trials evaluating the addition of Dara to lenalidomide and dexamethasone or bortezomib, melphalan, and prednisone,87,88 the advantage in survival outcomes seen for the general population was less clear, probably related to the fact that the HR population was underrepresented (∼10%). However, a meta-analysis of 6 phase 3 trials comparing backbone regimens with the same regimen plus daratumumab, including 3 for newly diagnosed transplant-eligible and non-transplant eligible (NTE) patients, showed that incorporation of daratumumab may be associated with improved PFS among patients with HRMM.89 In the SWOG-1211 phase 2 randomized study, the addition of elotuzumab to VRd as induction and maintenance therapy for HR patients with ND MM failed to improve their PFS compared with VRd.8 However, both study groups had improved PFS compared with statistical estimates, suggesting that a continuous treatment with PIs and IMiDs may be beneficial in HRMM. More intensive quadruplets, including monoclonal antibodies (MoAbs) and second-generation PIs and IMiDs combinations, are under investigation in phase 2 and 3 trials; preliminary results in some showed very high rates of high-quality responses and MRD negativity.90
Differently from fit patients, in those with poor Performance Status (PS) or frailty, the treatment should first not harm and the goal of therapy should be safety and quality of life rather than the depth of the response.91 Data from frailty-tailored treatments are still limited. The new standards of care including Dara frontline87,88 and VRd86 turned out to be feasible also in those older than 75 years of age, without a detrimental effect of age, and even in frail patients.92-94 However, expert opinion dose modification guidelines are available to adjust treatment based on patient fitness,8,77,82,93 considering both limited induction duration, lower drug doses, steroid-sparing regimens, and more tailored treatments.
Clinical case 3: dynamic risk evaluation and treatment of relapsed/refractory HRMM
A 68-year-old man with a previously established diagnosis of ISS III immunoglobulin G/λ MM and a HR characterized by the cosegregation of t(4;14), with del(17p) and P53 mutation (double-hit MM) was referred to us because of the persistence of MRD+ CR after first-line treatment with VTd induction + ASCT + VTd consolidation and len maintenance. Given the HR status and MRD positivity, a complete reevaluation of the disease was performed, detecting a BM PC infiltration of 20%, FISH unchanged, small monoclonal component (MC) (1 g/dL), and PET/CT and axial magnetic resonance imaging positive for diffuse BM involvement, without new lytic lesions. Biochemical early relapse was established.
Dynamic definition of HRMM and value of MRD
Regardless of baseline prognostic factors, risk is dynamic, with response and/or resistance to initial treatment being of utmost importance. In this setting, it is well known that early relapses (ie, within the first year after ASCT) and primary refractory disease should be considered as very HR factors.95,96
More recently, the importance of the depth of the response, and in particular the achievement of MRD negativity, that should be sustained over time, was highlighted, being associated with a longer PFS and OS, whether after the first line of therapy or at relapse.11 Current data show that the best way to overcome HR disease is to sustain MRD negativity,97 with the prognosis of HR patients achieving sustained MRD negativity getting very close to that of standard risk, meaning that the quality of the response may supersede the baseline risk factors, whereas on the contrary standard-risk patients may switch to the HR group if maintaining a persistent MRD positivity. Still, uncertainties remain on the long-term outcomes of sustained MRD− HR patients, as well as the probability to achieve this goal with respect to SR. Therefore, treatment selection in HR patients should be adapted to achieve MRD negativity, with the deepest possible level (currently 10−5 per IMWG recommendation; this threshold may change in the near future)98 and early therapeutic interventions can be proposed in case of persistent MRD positivity, even without biochemical/symptomatic relapses (Table 2). Also, at the time of the relapse, risk should be reevaluated, in light of the possible clonal evolution of the disease.99
Back to the clinical case
HR patients with early biochemical relapse after persistent MRD positivity represent an unmet medical need, and their enrollment into clinical studies exploring innovative approaches is highly recommended. At the time the patient was referred to us, at our center the CARTITUDE-4 phase 3 study comparing B-cell maturation antigen-targeting CAR-T therapy (cilta-cel) with Dara-based triplet combinations in patients with early relapse after ASCT was open to the enrollment. The patient was screened and randomized in the CAR-T arm. He is currently in CR and sustained MRD negativity after 18 months from CAR-T infusion.
Best available options for relapsed/refractory patients
In the past few years, several randomized trials showed the superiority of triplet combinations of PIs, IMiDs, and MoAbs and new classes of agents over the doublet backbone5,7 in both lenalidomide naïve/sensitive and refractory patients; the superiority in terms of survival outcomes of the triplets has been usually confirmed also in HR patients, albeit less pronounced than standard risk, showing an improvement and not an abrogation of the unfavorable impact of genetic alterations (Table 5).100-105 For this reason, no regimen is more uniquely suited than another in HR patients. Among the triplets in len-naïve patients,101,105-108 the longest PFS is afforded by Dara-len-dex (26.8 months),101 especially in patients achieving sustained MRD negativity105; however, the PFS in HR patients was almost one-half of that reported for the general population. In len-refractory patients, several PI- or pom-based triplets, combined with each other or with MoAbs, are available (Table 5) and showed benefit for HR, despite data being less mature than the previous mentioned len-based therapies.101,109-111 Also, the addition of chemotherapeutic agents may be of help for patients who are experiencing a rapid, aggressive relapse, particularly with characteristics of paraskeletal/EMD. In the past, HR patients at first or second relapse were considered for ASCT112; however, allogeneic transplant is often an exclusion criterion for subsequent clinical trials with newer agents.
Outcomes of the current approved triplet combinations for relapsed/refractory MM in genomic high-risk patients
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | No. HR patients (%) . | PFS rates . | MRD− (%) . |
|---|---|---|---|---|---|---|
| CANDOR110 | D-Kd vs Kd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | t(4;14), t(14;16), or del(17p) | 74 (16) | Median PFS: NE (D-Kd) vs 15.8 mo (Kd) | — |
| ELOQUENT-3117 | Elo-Pd vs Pd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | ISS stage II or III and del(17p), t(4;14), t(14;16) | 27 (23) | Median PFS: 6.2 mo (HR) vs 10.3 mo (SR) (Elo-Pd) 2.2 mo (HR) vs 5.2 mo (SR) (Pd) | — |
| CASTOR100,105 | D-Vd vs Vd | Randomized, open-label, controlled phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 91 (18) | Median PFS: 12.6 mo (HR) vs 16.6 mo (SR) (D-Vd) 6.2 mo (HR) vs 6.6 mo (SR) (Vd) | 15% (HR) vs 13% (SR) (D-Vd) 0 (HR) vs 3% (SR) (Vd) |
| OPTIMISMM109 | PVd vs Vd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 110 (20) | Median PFS: 8.44 mo (HR) vs 11.2 mo (ITT) (PVd) 5.32 mo (HR) vs 7.1 (ITT) (Vd) | — |
| POLLUX101,105 | D-Rd vs Rd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 65 (11) | Median PFS: 26.8 mo (HR) vs 52.0 mo (SR) (D-Rd) 8.3 mo (HR) vs 18.6 mo (SR) (Rd) | 29% (HR) vs 35% (SR) (D-Rd) 3% (HR) vs 9% (SR) (Rd) |
| ASPIRE102 | KRd vs Rd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 100 (13) | Median PFS: 23.1 mos (HR) vs 29.6 mo (SR) (KRd) 13.9 mo (HR) vs 19.5 mo (SR) (Rd) | — |
| ENDEAVOR118 | Kd vs Vd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 210 (23) | Median PFS: 8.8 mo (HR) vs NE (SR) (Kd) 6.0 mo (HR) vs 10.2 mo (SR) (Vd) | — |
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | No. HR patients (%) . | PFS rates . | MRD− (%) . |
|---|---|---|---|---|---|---|
| CANDOR110 | D-Kd vs Kd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | t(4;14), t(14;16), or del(17p) | 74 (16) | Median PFS: NE (D-Kd) vs 15.8 mo (Kd) | — |
| ELOQUENT-3117 | Elo-Pd vs Pd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | ISS stage II or III and del(17p), t(4;14), t(14;16) | 27 (23) | Median PFS: 6.2 mo (HR) vs 10.3 mo (SR) (Elo-Pd) 2.2 mo (HR) vs 5.2 mo (SR) (Pd) | — |
| CASTOR100,105 | D-Vd vs Vd | Randomized, open-label, controlled phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 91 (18) | Median PFS: 12.6 mo (HR) vs 16.6 mo (SR) (D-Vd) 6.2 mo (HR) vs 6.6 mo (SR) (Vd) | 15% (HR) vs 13% (SR) (D-Vd) 0 (HR) vs 3% (SR) (Vd) |
| OPTIMISMM109 | PVd vs Vd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 110 (20) | Median PFS: 8.44 mo (HR) vs 11.2 mo (ITT) (PVd) 5.32 mo (HR) vs 7.1 (ITT) (Vd) | — |
| POLLUX101,105 | D-Rd vs Rd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 65 (11) | Median PFS: 26.8 mo (HR) vs 52.0 mo (SR) (D-Rd) 8.3 mo (HR) vs 18.6 mo (SR) (Rd) | 29% (HR) vs 35% (SR) (D-Rd) 3% (HR) vs 9% (SR) (Rd) |
| ASPIRE102 | KRd vs Rd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 100 (13) | Median PFS: 23.1 mos (HR) vs 29.6 mo (SR) (KRd) 13.9 mo (HR) vs 19.5 mo (SR) (Rd) | — |
| ENDEAVOR118 | Kd vs Vd | Randomized, open-label, controlled, phase 3, RRMM (PFS) | del(17p), t(4;14), t(14;16) | 210 (23) | Median PFS: 8.8 mo (HR) vs NE (SR) (Kd) 6.0 mo (HR) vs 10.2 mo (SR) (Vd) | — |
D, daratumumab; Elo, elotuzumab; HR, high-risk; ITT, intention-to-treat; Kd, carfilzomib, dexamethasone; KRd, carfilzomib, lenalidomide, dexamethasone; NE, not estimable; NR, not reached; Pd, pomalidomide, dexamethasone; PVd, pomalidomide, bortezomib, dexamethasone; Rd, lenalidomide, dexamethasone; SR, standard risk; Vd, bortezomib, dexamethasone.
Little information is available on the efficacy of newer drugs, such as the oral inhibitor of exportin-1 Selinexor, or immunotherapy directed anti B-cell maturation antigen, such as belantamab mafodotin or bispecific MoAbs, alone or in combination, CAR-T cell therapy, in the specific HR population113; nevertheless, the capability of these newer approaches to induce relatively high rates of MRD negativity, in particular if applied early in the course of the disease, make them attractive and potentially a better way to pursue the goal of eradicating all malignant cells required in the context of aggressive disease. Additional promising agents, such as melphalan flufenamide, the first-in-class peptide drug conjugate, demonstrated substantial activity in patients with EMD.114 Results from ongoing studies of all of these new therapies in patients with HR disease are eagerly awaited.
First attempts to tailor treatment on risk assessment and integration with response-adapted approach
Randomized trials to date have not tailored therapy upon risk at study entry but have subsequently analyzed HR subgroups, in either planned or post hoc analyses, burdened by a small sample size and subsequently a reduced statistical power. Also, the evolving definition of HR patients complicates the design of risk-adapted clinical trials. In the past few years, attempts to address this have been made, with several phase 2 and 3 trials being recently presented/published and others ongoing, in different disease phases and based on different risk assessment/definition, showing at least the feasibility of such studies, even in a multicentric context (Table 6). Table 6 reports the currently ongoing prospective studies dedicated to HR patients.
Selected published/ongoing/planned clinical trials specifically dedicated to patients with high-risk ND MM according to prespecified different definitions
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | Results . |
|---|---|---|---|---|
| OPTIMUM57,119 | Dara-CVRd vs VRd | Phase 2b, first-line TE and TNE NDMM (MRD 100 d post-ASCT and PFS) | Two or more of: t[4;14], or t[14;16], t(14;20), del(1p32) gain(1q) or del(17p), HR-GEP, PCL (>20% cPCs) | 93% ORR, 52% CRs, 35% VGPRs, 5% PR MRD 50% |
| UK-MRA Myeloma XV (RADAR) (EudraCT: 2019- 001258-25) | Cy-PI-RD + ASCT followed by len ± PI ± Isa/12-mo Isa* | Phase 2, first-line TE and TNE NDMM (MRD and response) | t(4;14), t(14;16), t(14;20), del(17p), gain(1q) | Ongoing study |
| GMMG-CONCEPT90 | Isa-KRd in induction, consolidation, and maintenance ± ASCT | Phase 2, TE (arm A) and TNE (arm B) NDMM (MRD− 10−5 postconsolidation) | del17p or t(4;14) or t(14;16) or >3 copies 1q21 and ISS 2 or 3 stage disease | Interim analysis on 50 pts: 46 (A), 4 (B) ORR, ≥PR: 100%, ≥VGPR: 90%, CR/sCR: 46% MRD+: 20/33 (61%), MRD−: 11/33 (33%) |
| IRD Study (Nordic Myeloma Study Group) (HR-Maintenance Arm)120 | IRd induction and consolidation followed by IR maintenance (HR arm) | Phase 2, TE NDMM (MRD <0.01%) | t(4;14), del(17p) (60%), t(14;16), t(14;20), gain(1q) | Ongoing study |
| ANTARES EMN19 (NCT04166565) | CyBorD ± ASCT | Phase 2, NDMM or first relapse MM with EMD (≥CR) | EMD associated with high LDH level, del(17p) and HR-GEP | Ongoing study |
| SWOG 12119 | VRd vs VRd-Elo | Phase 2, TNE NDMM (PSF) | HR-GEP, t(14;16), t(14;20), del (17p), amp(1q21), primary PCL, or elevated serum LDH (≥2 × ULN) | Median FU 53 mo PFS 33.6 vs 31.5 mo (P = .449) OS NR vs 68 mo (P = .239) ORR 88% (44) vs 83% (39) ≥CR 6 vs 2.1% |
| EMN12121 | KRd ± ASCT followed by KR maintenance | Phase 2, no-randomized, TE and TNE pPCL patients (PFS) | (del(17p), t(4;14), t(14;16), del(1p), ampl(1q), ISS stage 3; elevated LDH | 14/15 pts ≤65 y received the planned 4 cycled of induction (1/15 off protocol for PD) ORR ≥ PR 93% ORR ≥ VGPR 80% (≥ CR 33%) (13% PR, 47% VGPR, 20% CR, 13% sCR) 0% mortality during induction No discontinuation due to toxicity AEs only first cycle KRd (decreased thereafter) |
| Intergroupe Francophone du Myelome 2018-04 (NCT03606577) | Dara-KRd for induction and consolidation + double ASCT | Phase 2, nonrandomized, NDMM TE | del(17p), or t(14;16) or t(4;14) | Ongoing study |
| Trial . | Regimen . | Study design (primary endpoint) . | Study definition of HR . | Results . |
|---|---|---|---|---|
| OPTIMUM57,119 | Dara-CVRd vs VRd | Phase 2b, first-line TE and TNE NDMM (MRD 100 d post-ASCT and PFS) | Two or more of: t[4;14], or t[14;16], t(14;20), del(1p32) gain(1q) or del(17p), HR-GEP, PCL (>20% cPCs) | 93% ORR, 52% CRs, 35% VGPRs, 5% PR MRD 50% |
| UK-MRA Myeloma XV (RADAR) (EudraCT: 2019- 001258-25) | Cy-PI-RD + ASCT followed by len ± PI ± Isa/12-mo Isa* | Phase 2, first-line TE and TNE NDMM (MRD and response) | t(4;14), t(14;16), t(14;20), del(17p), gain(1q) | Ongoing study |
| GMMG-CONCEPT90 | Isa-KRd in induction, consolidation, and maintenance ± ASCT | Phase 2, TE (arm A) and TNE (arm B) NDMM (MRD− 10−5 postconsolidation) | del17p or t(4;14) or t(14;16) or >3 copies 1q21 and ISS 2 or 3 stage disease | Interim analysis on 50 pts: 46 (A), 4 (B) ORR, ≥PR: 100%, ≥VGPR: 90%, CR/sCR: 46% MRD+: 20/33 (61%), MRD−: 11/33 (33%) |
| IRD Study (Nordic Myeloma Study Group) (HR-Maintenance Arm)120 | IRd induction and consolidation followed by IR maintenance (HR arm) | Phase 2, TE NDMM (MRD <0.01%) | t(4;14), del(17p) (60%), t(14;16), t(14;20), gain(1q) | Ongoing study |
| ANTARES EMN19 (NCT04166565) | CyBorD ± ASCT | Phase 2, NDMM or first relapse MM with EMD (≥CR) | EMD associated with high LDH level, del(17p) and HR-GEP | Ongoing study |
| SWOG 12119 | VRd vs VRd-Elo | Phase 2, TNE NDMM (PSF) | HR-GEP, t(14;16), t(14;20), del (17p), amp(1q21), primary PCL, or elevated serum LDH (≥2 × ULN) | Median FU 53 mo PFS 33.6 vs 31.5 mo (P = .449) OS NR vs 68 mo (P = .239) ORR 88% (44) vs 83% (39) ≥CR 6 vs 2.1% |
| EMN12121 | KRd ± ASCT followed by KR maintenance | Phase 2, no-randomized, TE and TNE pPCL patients (PFS) | (del(17p), t(4;14), t(14;16), del(1p), ampl(1q), ISS stage 3; elevated LDH | 14/15 pts ≤65 y received the planned 4 cycled of induction (1/15 off protocol for PD) ORR ≥ PR 93% ORR ≥ VGPR 80% (≥ CR 33%) (13% PR, 47% VGPR, 20% CR, 13% sCR) 0% mortality during induction No discontinuation due to toxicity AEs only first cycle KRd (decreased thereafter) |
| Intergroupe Francophone du Myelome 2018-04 (NCT03606577) | Dara-KRd for induction and consolidation + double ASCT | Phase 2, nonrandomized, NDMM TE | del(17p), or t(14;16) or t(4;14) | Ongoing study |
MRD− only.
AEs, adverse events; ASCT, autologous stem-cells transplantation; CR, complete response; CyBorD, cyclophosphamide, bortezomib and dexamethasone; Cy-PI-RD, cyclophosphamide, pomalidomide, lenalidomide, and dexamethasone; CVRd, bortezomib, lenalidomide, cyclophosphamide, and dexamethasone; Dara, daratumumab; DSMM, Deutsche Studiengruppe MM; Elo, elotuzumab; EMD, extra-medullary disease; GEP, gene expressing profile; HR, high-risk; IRd, isatuximab, lenalidomide, and dexamethasone; Isa, isatuximab; KRd, carfilzomib, lenalidomide, and dexamethasone; LDH, lactate dehydrogenase; len, lenalidomide; MRD, minimal residual disease; NR, not reported; ORR, overall response rate; OS, overall survival; PCL, plasma cell leukemia; Pd, pomalidomide and dexamethasone; PFS, progression-free survival; PR, partial response; sCR, stringent complete response; TE, transplant eligible; TNE, transplant noneligible; TPP, time to progression; ULN, upper limit of normal; VGPR, very good partial response; VRd, bortezomib, lenalidomide and dexamethasone.
Because it was clearly shown that the achievement and sustenance of MRD negativity supersedes the genetic risk, another way to plan tailored trials is to adapt treatment (in terms of choice of drugs and duration) on MRD status, integrating the baseline with the dynamic risk assessment. Several prospective randomized clinical trials are currently ongoing with this design.
Conclusion
HRMM represents a composed group of patients (15%-20%), characterized by reduced survival from either to the biology of the tumor or some form of frailty or to suboptimal/lack of response to therapy. Identifying different risk factors for aggressive disease is crucial to succeed with and manage this difficult-to-treat population of patients; efforts are ongoing for a more precise, reproducible, and universal definition. In biologic HRMM, the goal of treatment should be to achieve and sustain MRD negativity, inside and outside the BM. In patients with frailty, therapy should be adapted to reduce toxicity and improve quality of life. So far, treatment has rarely been adapted according to risk stratification, but in the past few years some attempts in this direction have been successfully made. The combination of risk- and MRD-adapted treatment strategy may represent the optimal approach.
Authorship
Contribution: E.Z. performed bibliography research and analysis of published data and wrote the manuscript; S.B. supported the bibliographic research and manuscript preparation; and M.C. contributed to paper writing and critically revised the paper.
Conflict-of-interest disclosure: E.Z. receives honoraria from Janssen, Bristol-Myers Squibb, Amgen, and Takeda. M.C. receives honoraria from Janssen, Celgene, Amgen, Bristol-Myers Squibb, Takeda, AbbVie, Sanofi, and Adaptive Biotechnologies, and is a member of Janssen's and Celgene's Speaker's Bureau. S.B. declares no competing financial interests.
Correspondence: Elena Zamagni, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Istituto di Ematologia “Seràgnoli,” Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna, Via Massarenti 9, 40138, Bologna, Italy; e-mail: e.zamagni@unibo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal