Abstract
Despite recent advances, multiple myeloma remains an incurable disease for most patients, and initial remission will be followed by relapses requiring therapy. For many, there will be several remissions and relapses until resistance develops to all available therapies. With the introduction of several new agents, myeloma treatment has changed drastically, and there are new options for the management of relapsed or refractory disease, including new drug classes with distinct mechanisms of action and cellular therapies. However, resistance to major drug classes used in first-line remains the most critical factor for the choice of treatment at relapse. Continuous lenalidomide-based therapy is used extensively at first-line, and resistance to lenalidomide has become the key factor for the choice of salvage therapy. Daratumumab is increasingly used in first-line, and soon patients that relapse while on daratumumab will become a common challenge. Three-drug regimens are the standard approach to manage relapsed disease. Adding drugs with new mechanisms of activity can improve outcomes and overcomes class resistance, but, until now, while biology is important, it can offer only limited guidance for the choice of therapy.
Introduction
Despite recent advances, multiple myeloma remains an incurable disease for most patients. For many, there will be several remissions and relapses until resistance develops to available therapies. Myeloma treatment options have increased and, while advances in primary therapy have improved outcomes, they have also generated new challenges in the management of relapsing disease, illustrated in the recent recommendations by the European Hematology Society (EHA)/European Society for Medical Oncology (ESMO) and the International Myeloma Working Group (IMWG).1,2 A critical challenge remains the availability of the new treatments in many countries; delays in the approvals and extremely high costs have resulted in significant inequalities in access to new therapies.
According to IMWG definitions,3,4 refractoriness to a specific agent is defined as relapse/progression while on treatment or, arbitrarily, within <60 days from the last dose of the drug. The clinical trials that evaluated the new drugs and combinations (by inclusion/exclusion criteria) were designed based on these definitions. However, these criteria cannot capture the underlying biology of the relapse or the mechanisms of drug resistance. In addition, these definitions were developed at a time when maintenance therapy was not standard and many of the contemporary drug classes were not available, or only a single agent from a drug class was in clinical use. When treatment options were fewer, the definition of relapsing myeloma required “clinically active disease, not just biochemical M-protein presence or increment.”3 However, more data5-9 support that such biochemical relapses should probably be treated earlier than later, while even the definition of symptomatic myeloma now includes biomarkers of malignancy.10
Clinical case 1
A.B., a 58-year-old woman, was diagnosed with IgGκ myeloma International Staging System (ISS) stage 3, 3 years ago. Fluorescence in situ hybridization (FISH) studies revealed t(4;14) and amp1q21 but no del17p. After induction with bortezomib, lenalidomide, and dexamethasone (VRd), she received a single autologous stem cell transplant (ASCT) and achieved a very good partial response (VGPR) (positive immunofixation and nonvisible spike) followed by lenalidomide maintenance, but after 2 years her monoclonal protein started rising and now is ∼0.7 gr/dl. She remains asymptomatic.
Management of patients who have received 1 prior line of therapy and are lenalidomide-resistant
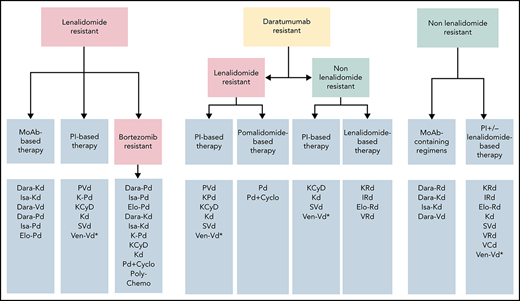
Resistance to 1 or more drugs is the most critical among the factors affecting the choice of therapy in a patient presenting with disease progression (Figures 1 and 2); this is also the critical decision factor in EHA/ESMO1 and IMWG2 guidelines. Today, as part of their first-line therapy, most patients receive continuous therapy with lenalidomide, either as maintenance (single agent at lower doses, as A.B.) or as part of continuous therapy (at the tolerated dose, without or with steroids); some patients may also receive maintenance with bortezomib,11 either alone or with steroids or with lenalidomide or thalidomide.12 In the setting of relapse/progression in a patient receiving lenalidomide, data support that resistance at lower doses predicts resistance at higher doses.13,14 There is no randomized data that increasing the dose of lenalidomide can overcome resistance; however, the addition of dexamethasone to the regimen (switching to lenalidomide-dexamethasone [Rd]) may be associated with some efficacy in retrospective analyses.15,16 Longer duration of lenalidomide therapy before progression may be associated with a higher probability of a better outcome on pomalidomide-based therapy,13,14 but this may also be relevant for bortezomib and carfilzomib.17 Finally, a longer period between different immunomodulatory imide drugs (IMiDs) (IMiD-free) may result in better outcomes when pomalidomide-based therapy is given.14 Class switch is important to overcome resistance to lenalidomide, but pomalidomide may also be active in lenalidomide-refractory patients, especially with the addition of a third non cross-resistant agent.
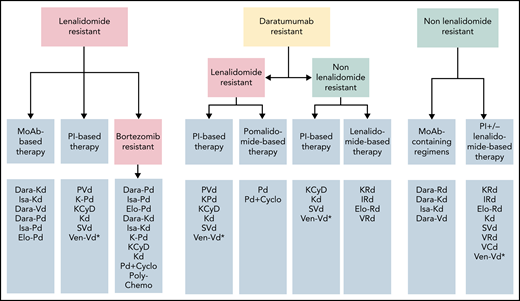
Approach to choice of regimens at first relapse according to resistance to agents used at first line. *Not approved, could be considered only if t(11;14) is present.
Approach to choice of regimens at first relapse according to resistance to agents used at first line. *Not approved, could be considered only if t(11;14) is present.
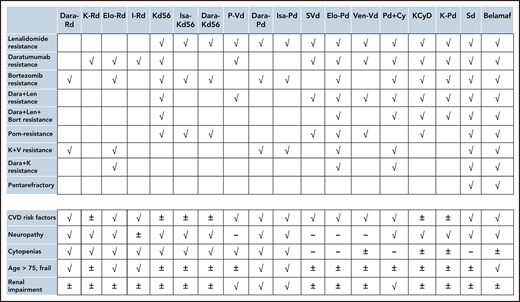
Potential options for patients with various characteristics (prior resistance and patient-related factors). √: Go, -: No-Go, ±: may be considered cautiously. The choices in the upper and lower panel may be combined. Len, lenalidomide; Bor, bortezomib; Dara, daratumumab; K, carfilzomib; CVD, cardiovascular; Dara, Rd, daratumumab, lenalidomide, dexamethasone; K-Rd, carfilzomib, lenalidomide, dexamethasone; Elo-Rd, elotuzumab, lenalidomide, dexamethasone; I-Rd, ixazomib, lenalidomide, dexamethasone; Isa-Kd56, isatuximab, carfilzomib, dexamethasone; Dara-Kd56, daratumumab, carfilzomib, dexamethasone; PVd, pomalidomide, bortezomib, dexamethasone; Dara, Pd, daratumumab, pomalidomide, dexamethasone; Isa, Pd, isatuximab, pomalidomide, dexamethasone; Elo, Pd, elotuzumab, pomalidomide, dexamethasone; S-Vd, selinexor, bortezomib, dexamethasone; Ven-Vd, venetoclax, bortezomib, dexamethasone; Pd-Cy, pomalidomide, dexamethasone, cyclophosphamide; KCyD, carfilzomib, cyclophosphamide, dexamethasone; K-Pd, carfilzomib, pomalidomide, dexamethasone; Sd, selinexor, dexamethasone; Belamaf, belantamab mafodotin.
Potential options for patients with various characteristics (prior resistance and patient-related factors). √: Go, -: No-Go, ±: may be considered cautiously. The choices in the upper and lower panel may be combined. Len, lenalidomide; Bor, bortezomib; Dara, daratumumab; K, carfilzomib; CVD, cardiovascular; Dara, Rd, daratumumab, lenalidomide, dexamethasone; K-Rd, carfilzomib, lenalidomide, dexamethasone; Elo-Rd, elotuzumab, lenalidomide, dexamethasone; I-Rd, ixazomib, lenalidomide, dexamethasone; Isa-Kd56, isatuximab, carfilzomib, dexamethasone; Dara-Kd56, daratumumab, carfilzomib, dexamethasone; PVd, pomalidomide, bortezomib, dexamethasone; Dara, Pd, daratumumab, pomalidomide, dexamethasone; Isa, Pd, isatuximab, pomalidomide, dexamethasone; Elo, Pd, elotuzumab, pomalidomide, dexamethasone; S-Vd, selinexor, bortezomib, dexamethasone; Ven-Vd, venetoclax, bortezomib, dexamethasone; Pd-Cy, pomalidomide, dexamethasone, cyclophosphamide; KCyD, carfilzomib, cyclophosphamide, dexamethasone; K-Pd, carfilzomib, pomalidomide, dexamethasone; Sd, selinexor, dexamethasone; Belamaf, belantamab mafodotin.
The currently approved regimens for lenalidomide-resistant patients, with available data from phase 3 studies, can be divided into regimens with and without monoclonal antibodies (ie, proteasome inhibitor-based [PI-based]) (Table 1; Figure 1). Anti-CD38 monoclonal antibody-containing regimens with a PI (both classes are noncross resistant to IMiDs) include daratumumab with bortezomib and dexamethasone (Dara-Vd),18 daratumumab or isatuximab with Kd56 (Dara-Kd5619 and Isa-Kd20). However, only a subset of patients in the respective trials was lenalidomide-refractory, and fewer after 1 prior line (Table 1). In the CANDOR study in the small subset of lenalidomide-refractory patients after 1 prior line of therapy, the hazard ratio (HR) was 0.11 (95% confidence interval [CI], 0.02-0.52).21 Based on the EQUULEUS study, daratumumab with carfilzomib has also been approved by the FDA with a dose for carfilzomib of 70 mg/m2 once per week (Dara-Kd70).22 In the IKEMA study, the HR for patients refractory to lenalidomide treated with Isa-Kd was 0.60 (95% CI, 0.34-1.06), and for those who were refractory to lenalidomide at last regimen was 0.69 (95% CI, 0.35-1.39).
Regimens for patients with lenalidomide-refractory disease
| . | Regimen . | Median prior lines of therapy . | Len-refractory n (% of total study population) . | mPFS for all population . | mPFS for first relapse . | mPFS, mo for len refractory . | PFS HR for len refractory . | mPFS, months for len refractory at first relapse or HR . | Approved therapy . |
|---|---|---|---|---|---|---|---|---|---|
| CASTOR | Dara-Vd | 2 (1-10) | 60 (24) | 16.7 | 27 | 7.8 | 0.44 | NR | YES after at least 1 prior line |
| Vd | 81 (33) | 7.1 | 7.9 | 4.9 | NR | ||||
| CANDOR | Dara-Kd56 | 2 (1-3) | 99 (32) | NE | NE (HR, 0.68) | NE | 0.474 | HR, 0.11 (0.02-0.52) | YES (FDA: after 1-3 prior lines; Dara-Kd56 & Dara-Kd70; EMA: after 1 prior line only Dara-Kd56) |
| Kd56 | 55 (36) | 15.8 | 11.1 | ||||||
| IKEMA | Isa-Kd56 | 2 (1-3) | 57 (32) | NE | NE (HR, 0.59) | NE | 0.60 (0.34-1.06) | HR, 0.69 for refractory to len at last line | YES (FDA: after 1-3 prior lines; EMA: after 1 prior line) |
| Kd56 | 42 (34) | 19.15 | 15.7 | ||||||
| APOLLO | Dara-Pd | 2 (1-5) | 120 (79) | 12.4 | 14.1 | 9.9 | 0.66 | NR | YES |
| Pd | 122 (80) | 6.9 | 12.6 | 6.5 | NR | ||||
| ICARIA | Isa-Pd | 3 (2-11) | 144 (94) | 11.5 | − | 11.4 | 0.59 | 11.6 vs 5.7 if Len-refractory at last line (HR, 0.50) | YES (after >2 lines of therapy) |
| Pd | 140 (92) | 6.5 | − | 5.59 | |||||
| ELOQUENT-3 | Elo-Pd | 3 (2-8) | 59 (98) | 10.3 | − | NR | NR | NR | YES (after >2 lines of therapy) |
| Pd | 55 (96) | 4.7 | − | NR | NR | NR | |||
| ENDEAVOR | Kd | 2 (1-3) | 113 (24) | 18.7 | 22.2 | 8.6 | 0.80 | <18 mo of prior len: 10.8 vs 6.7 mo | YES after at least 1 prior line |
| Vd | 122 (26) | 9.4 | 10.1 | 6.6 | |||||
| OPTIMISMM | Bort-Pd | 2 (1-3) | 200 (71) | 11.2 | 20.7 | NR | 0.65 | 17.6 vs 9.5; HR, 0.55 | YES (EMA: after at least 1 prior line that included lenalidomide) |
| Pd | 191 (69) | 7.1 | 11.6 | NR | |||||
| BOSTON | Sel-Vd | 1 (1-3) | 77 (39) | 13.93 | 16.62 | 9.59 | 0.63 | NR | YES (FDA only: after at least 1 prior line) |
| Vd | 77 (37) | 9.46 | 10.68 | 7.23 | NR | ||||
| BELLINI | Ven-Vd | 1 (1-3) | 38 (20) | 22.4 | 22.4 | NE | 0.75 | NR | NO |
| Vd | 27 (28) | 11.5 | 11.4 | 14.8 | NR |
| . | Regimen . | Median prior lines of therapy . | Len-refractory n (% of total study population) . | mPFS for all population . | mPFS for first relapse . | mPFS, mo for len refractory . | PFS HR for len refractory . | mPFS, months for len refractory at first relapse or HR . | Approved therapy . |
|---|---|---|---|---|---|---|---|---|---|
| CASTOR | Dara-Vd | 2 (1-10) | 60 (24) | 16.7 | 27 | 7.8 | 0.44 | NR | YES after at least 1 prior line |
| Vd | 81 (33) | 7.1 | 7.9 | 4.9 | NR | ||||
| CANDOR | Dara-Kd56 | 2 (1-3) | 99 (32) | NE | NE (HR, 0.68) | NE | 0.474 | HR, 0.11 (0.02-0.52) | YES (FDA: after 1-3 prior lines; Dara-Kd56 & Dara-Kd70; EMA: after 1 prior line only Dara-Kd56) |
| Kd56 | 55 (36) | 15.8 | 11.1 | ||||||
| IKEMA | Isa-Kd56 | 2 (1-3) | 57 (32) | NE | NE (HR, 0.59) | NE | 0.60 (0.34-1.06) | HR, 0.69 for refractory to len at last line | YES (FDA: after 1-3 prior lines; EMA: after 1 prior line) |
| Kd56 | 42 (34) | 19.15 | 15.7 | ||||||
| APOLLO | Dara-Pd | 2 (1-5) | 120 (79) | 12.4 | 14.1 | 9.9 | 0.66 | NR | YES |
| Pd | 122 (80) | 6.9 | 12.6 | 6.5 | NR | ||||
| ICARIA | Isa-Pd | 3 (2-11) | 144 (94) | 11.5 | − | 11.4 | 0.59 | 11.6 vs 5.7 if Len-refractory at last line (HR, 0.50) | YES (after >2 lines of therapy) |
| Pd | 140 (92) | 6.5 | − | 5.59 | |||||
| ELOQUENT-3 | Elo-Pd | 3 (2-8) | 59 (98) | 10.3 | − | NR | NR | NR | YES (after >2 lines of therapy) |
| Pd | 55 (96) | 4.7 | − | NR | NR | NR | |||
| ENDEAVOR | Kd | 2 (1-3) | 113 (24) | 18.7 | 22.2 | 8.6 | 0.80 | <18 mo of prior len: 10.8 vs 6.7 mo | YES after at least 1 prior line |
| Vd | 122 (26) | 9.4 | 10.1 | 6.6 | |||||
| OPTIMISMM | Bort-Pd | 2 (1-3) | 200 (71) | 11.2 | 20.7 | NR | 0.65 | 17.6 vs 9.5; HR, 0.55 | YES (EMA: after at least 1 prior line that included lenalidomide) |
| Pd | 191 (69) | 7.1 | 11.6 | NR | |||||
| BOSTON | Sel-Vd | 1 (1-3) | 77 (39) | 13.93 | 16.62 | 9.59 | 0.63 | NR | YES (FDA only: after at least 1 prior line) |
| Vd | 77 (37) | 9.46 | 10.68 | 7.23 | NR | ||||
| BELLINI | Ven-Vd | 1 (1-3) | 38 (20) | 22.4 | 22.4 | NE | 0.75 | NR | NO |
| Vd | 27 (28) | 11.5 | 11.4 | 14.8 | NR |
EMA, European Medicines Agency; FDA, Food and Drug Administration; NE, not evaluable/not reached; NR, not reported.
Combinations of monoclonal antibodies (daratumumab, isatuximab, or elotuzumab) with pomalidomide and dexamethasone (Dara-Pd,23 Isa-Pd,24 and Elo-Pd,25 respectively) were evaluated in patients exposed and mostly refractory to lenalidomide and bortezomib, but not at first relapse. In the APOLLO study (that compared Dara-Pd vs Pd), a small subgroup of patients (only 34 patients) was refractory to lenalidomide and bortezomib after 1 line of therapy.23
Nonmonoclonal antibody-containing regimens based on PIs, with activity in lenalidomide-resistant patients, include primarily pomalidomide combined with bortezomib-dexamethasone (Vd) (PomVd): this regimen was evaluated in lenalidomide-exposed and mostly lenalidomide-resistant patients (71% were refractory to lenalidomide). Other regimens include the doublet of carfilzomib 56 mg/m2 and dexamethasone (Kd56),26 and triplets such as panobinostat with Vd (Pano-Vd),27 selinexor with bortezomib and dexamethasone (SVd),28 and venetoclax with Vd (Ven-Vd).18,29 Data on the activity of selinexor with Vd (SVd)28 or Vd plus venetoclax30 in the setting of lenalidomide-refractory patients are limited (Table 1). SVd is approved by the Food and Drug Administration (FDA) but not the European Medicines Association (EMA), and the Ven-Vd combination has not been approved.
As shown in Table 1, the regimens containing monoclonal antibodies with second-generation PI (Dara-Kd56 and Isa-Kd56) show longer progression-free survival (PFS) with more favorable HR vs their comparator (Kd56) in the subset of lenalidomide-resistant patients. Both Dara-Kd56 and Isa-Kd56 have been approved for the treatment of patients exposed to at least 1 prior line of treatment (not necessarily including lenalidomide). When compared with combinations with monoclonal antibodies and Pd (all of which, Dara-Pd,23 Isa-Pd,24 and Elo-Pd,25 had similar PFS of about 10 to 12 months) also show more favorable results; however, these Pd plus monoclonal antibody regimens were evaluated in more heavily pretreated patients (most patients were double refractory), and this comparison may be unfair. Among lenalidomide-refractory patients after 1 prior treatment, data on PVd (a nonmonoclonal antibody-containing regimen) showed a favorable outcome (median PFS for PVd [n = 64] was 17.8 months vs 9.5 months and for Vd [n = 65]; HR, 0.55),31 but such data are lacking for the other regimens.
Other regimens with potential activity in lenalidomide-refractory patients that have not been evaluated in phase 3 studies include pomalidomide/dexamethasone with cyclophosphamide,32-37 or with carfilzomib (KPd).38-40 The combination of Kd (with carfilzomib at a weekly dose of 70 mg/m2) with (KCyd) or without (Kd) cyclophosphamide has been evaluated in a phase 2 study in relapsed/refractory (RR) patients with 1 to 3 prior lines41 of PFS in the IMiD-refractory population was 26.2 months for KCyd vs 7.7 months for Kd. In the phase 1/2 study, an all-oral combination of ixazomib, pomalidomide, and dexamethasone reported a response rate of ∼52%.42 A small phase 2 study has also evaluated the addition of cyclophosphamide to Rd in lenalidomide-refractory patients, with some activity.43
The studies above have used different inclusion and exclusion criteria (mainly regarding prior exposure to therapies, renal function, other comorbidities) while patients are generally fitter than in clinical practice; thus, their results cannot be extrapolated to every patient. Depending on the availability of the various drugs, PVd (which has mature data in second-line setting), Dara-Kd, and Isa-Kd are our primary choices (Figure 1); Dara-Vd seems less active than Dara-Kd or Isa-Kd. Subcutaneous over IV use of daratumumab may be more convenient.23,44,45 Use of bortezomib and carfilzomib are associated with different toxicities and logistical issues; there is no data that ixazomib could substitute bortezomib. Dara-Pd has been approved for patients who have at least 1 prior line of therapy containing a PI and lenalidomide and were lenalidomide-refractory, but Isa-Pd for patients who have at least 2 prior lines. A doublet of Kd56 may be an option for some patients but certainly inferior to a triplet of KD56 with a third agent. Additional considerations for choice of therapy include tolerability and specific toxicities as well as availability and costs (Figure 2). A doublet such as Vd alone is probably a poor choice for lenalidomide-refractory patients (and we do not recommend it), but triplets containing a Vd-backbone such as bortezomib with cyclophosphamide and dexamethasone (VCd) or bortezomib-melphalan-prednisone (VMP) may be an option for lenalidomide-refractory patients in a resource-poor setting. A.B. was treated with PVd, and achieved a VGPR but had to discontinue bortezomib due to neuropathy. Adding a non-neurotoxic PI (such as carfilzomib) or a monoclonal antibody to Pd could be an option, but such data are not available in this special setting, and we continued Pd without adding a third agent to substitute for bortezomib.
Clinical case 2
C.D., a 69-year-old man, was diagnosed with IgAλ myeloma ISS-3, presenting with anemia and bone lesions. FISH studies showed the presence of t(4;14), del17p, and del13q. He started therapy with VRd. After initial clinical improvement (increase in hemoglobin and major improvement in bone pain) and a hematologic response (achieved VGPR), IgA is increasing and hemoglobin is dropping.
Management of patients who are both lenalidomide- and bortezomib-resistant
The treatment of patients such as C.D. is particularly challenging. Early progression on primary therapy is associated with poor outcome46-48 even in the era of new therapies. Patients who develop resistance at the time of initial therapy to both lenalidomide and bortezomib have significantly fewer options at relapse, and only a few have been included in clinical trials of the novel triplets. A reasonable approach to manage the disease of C.D. is to combine agents that have no cross-resistance or that could at least partly overcome resistance to lenalidomide and bortezomib. Monoclonal antibody-based regimens with either pomalidomide/dexamethasone or second-generation PI (carfilzomib) could be options. In the APOLLO study (that compared Dara-Pd to Pd), 42% were refractory to both lenalidomide and bortezomib with poor PFS (7.7 vs 6.1 months; HR, 0.74), but few were refractory to both after 1 line of therapy. In ICARIA, about 70% of patients were refractory to both lenalidomide and bortezomib (but after at least 2 lines of therapy), and PFS was 11.2 months vs 4.8 months (HR, 0.58) for Isa-Pd vs Pd. In ELOQUENT-3, 70% were also double refractory; Elo-Pd was associated with an HR for PFS of 0.56 compared with Pd.25 In IKEMA and CANDOR studies, such double-refractory patients were few, and no subgroup data are available. The efficacy of D-Kd and Isa-Kd cannot be inferred by the available data in double refractory patients, although their efficacy in PI-exposed patients was better than Kd56 alone, but marginal in PI refractory patients (HR, 0.84 with dara-Kd56)19 while for Isa-Kd56 the HR for patients with previous proteasome plus IMiD treatment at last regimen was 0.78.20 Many patients may have never been exposed to an alkylating agent. Regimens containing an alkylating agent such as cyclophosphamide, melphalan, or bendamustine may be options. These alkylating agents can be combined with Pd32-37 or Kd (KCyd, Benda-Kd).49-51 Depending on specific patient characteristics and comorbidities (neuropathy, renal function, cytopenias) (Figure 2), some regimens may fit better to a specific patient. Ideally, patients like C.D. should be given the option to participate in a clinical trial with new combinations and should be managed like patients that developed resistance after more lines of therapy. For this patient, our primary option was anti-CD38-based therapy with Pd (at that time, Dara-Pd was available but now also EMA- and FDA-approved for patients after ≥1 line of therapy containing lenalidomide and bortezomib). In fit patients, polychemotherapy regimens52 may be an alternative as a bridge to more targeted therapy.
Clinical case 3
E.F. (male) was diagnosed with IgGκ myeloma ISS-2 at the age of 73 years. He presented with anemia, bone pain, spinal compression fracture, and renal dysfunction. FISH studies showed the presence of t(4;14) and amp1q21. He started therapy with VCd with rapid renal function improvement and achieved a VGPR. He completed 8 cycles of therapy and discontinued bortezomib due to peripheral neuropathy. After 2 years, immunofixation became positive for IgGκ, and within 6 months, monoclonal spike reached 1.2 gr/dl and hemoglobin started to drop.
Management of patients who are not lenalidomide-resistant
For patients who relapse after primary therapy that did not include lenalidomide (eg, patients treated with bortezomib-based regimen, usually of fixed duration, as E.F.) or relapse/progress several months (at least 6 months but preferably longer) after last lenalidomide dose, second-line therapy can include an Rd-based regimen such as carfilzomib with Rd (KRd),53 daratumumab with Rd (Dara-Rd),54 ixazomib with Rd (IRd),55 or elotuzumab with Rd (Elo-Rd)56 (Table 2). Based on PFS outcomes and hazard ratios when compared with Rd, Dara-Rd is the preferable regimen, especially for standard-risk patients at first relapse (median PFS at first relapse was 53.3 months for Dara-Rd vs 19.6 months for Rd; HR, 0.42) while at 4-year follow-up, the median PFS2 was 53.3 months vs 31.6 months for Rd (HR, 0.54).57 Although OS data have not been presented yet, it is likely that Dara-Rd is associated with an OS benefit. KRd53,58,59 is associated with a significant improvement of PFS and OS in the first relapse. Elo-Rd56,60 improved PFS mostly in patients with a longer time from diagnosis (median of 3.5 years in the study),61 but OS benefit was observed mostly among patients with 2 to 3 prior lines.62 PFS benefit with IRd was mostly seen in patients with 2 to 3 prior lines; there was no PFS benefit among patients with 1 prior line and prior transplant63 and neither overall survival (OS) benefit.64
Results of phase 3 randomized studies which evaluated regimens for patient with 1 to 3 prior lines of therapy
| . | Dara-Rd . | Rd . | K-Rd . | Rd . | Elo-Rd . | Rd . | Ixa-Rd . | Rd . | Kd . | Vd . | Dara-Vd . | Vd . | Dara-Kd . | Kd . | Isa-Kd . | Kd . | SVd . | Vd . | Ven-Vd . | Vd . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median of prior lines of therapy in the study (range) | 1 (1-11) | 2 (1-3) | 2 (1-4) | 1 (1-3) | 2 (1-3) | 2 (1-10) | 2 (1-3) | 1 (1-3) | 1 (1-3) | 2 (1-3) | ||||||||||
| Exclusion per prior therapy | Lenalidomide- resistant | Lenalidomide- resistant and bortezomib- resistant if at last line | Lenalidomide- resistant | Lenalidomide- or bortezomib- resistant | Bortezomib- resistant | Bortezomib- resistant | previous resistance to carfilzomib, or refractory to anti-CD38 | previous treatment with carfilzomib, or refractory to anti-CD38 | Bortezomib- resistant | Bortezomib- resistant | ||||||||||
| ITT population | 283 | 286 | 396 | 396 | 321 | 325 | 360 | 362 | 464 | 465 | 251 | 247 | 312 | 154 | 179 | 123 | 195 | 207 | 194 | 97 |
| PFS | 46 | 17.5 | 26.3 | 17.6 | 19.4 | 14.9 | 20.6 | 14.7 | 18.7 | 9.4 | 16.7 | 7.1 | NE | 15.8 | NE | 19.1 | 13.9 | 9.4 | 22.4 | 11.5 |
| HR (95% CI) | 0.42 (0.33-0.52) | 0.69 (0.57-0.83) | 0.71 (0.59-0.86) | 0.74 (0.59-0.94) | 0.53 (0.44-0.65) | 0.31 (0.25-0.39) | 0.63 (0.46-0.85) | 0.53 (0.32-0.89) | 0.70 (0.53-0.93) | 0.63 (0.44-0.90) | ||||||||||
| 1 prior line | 149 | 146 | 184 | 157 | 151 | 159 | 212 | 213 | 231 | 229 | 247 | 251 | 144 | 70 | 80 | 55 | 99 | 99 | 91 | 44 |
| PFS | 53.3 | 19.6 | 29.6 | 17.6 | NA | NA | 20.6 | 16.6 | 22.2 | 10.1 | 27 | 7.9 | NA | NA | 22.4 | 11.4 | ||||
| HR | 0.42 (0.30-0.57) | 0.713 (0.532-0.957 | 0.77 (0.59-1.01) | 0.882 (0.65-1.197) | 0.447 (0.330-0.606) | 0.22 (0.15-0.32) | 0.68 (0.40-1.14) | 0.589 (0.309-1.123) | 0.63 (0.41-0.96) | 0.75 (0.45-1.26) | ||||||||||
| 2-3 prior lines | 123 | 118 | 212 | 239 | 170 | 166 | 148 | 149 | 232 | 233 | 106 | 107 | 179 | 87 | 99 | 68 | 96 | 108 | 103 | 53 |
| PFS | NA | NA | 25.8 | 16.7 | NA | NA | NE | 12.9 | 14.9 | 8.4 | NA | NA | NA | NA | NA | NA | NA | NA | NE | 14 |
| HR (95% CI) | 2 prior lines: 0.39 (0.26-0.58) | 0.720 (0.561-0.923) | 0.68 (0.53-0.87) | 0.58 (0.401-0.838) | 0.604 (0.466-0.783) | 2 prior lines: 0.46 (0.30-0.72) | 0.61 (0.42-0.88) | 0.479 (0.294-0.778) | 2 prior lines: 0.65 (0.40-1.07) | 0.54 (0.33-0.88) | ||||||||||
| 3 prior lines: 0.48 (0.25-0.94) | 3 prior lines: 0.60 (0.33-1.07) | |||||||||||||||||||
| . | Dara-Rd . | Rd . | K-Rd . | Rd . | Elo-Rd . | Rd . | Ixa-Rd . | Rd . | Kd . | Vd . | Dara-Vd . | Vd . | Dara-Kd . | Kd . | Isa-Kd . | Kd . | SVd . | Vd . | Ven-Vd . | Vd . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median of prior lines of therapy in the study (range) | 1 (1-11) | 2 (1-3) | 2 (1-4) | 1 (1-3) | 2 (1-3) | 2 (1-10) | 2 (1-3) | 1 (1-3) | 1 (1-3) | 2 (1-3) | ||||||||||
| Exclusion per prior therapy | Lenalidomide- resistant | Lenalidomide- resistant and bortezomib- resistant if at last line | Lenalidomide- resistant | Lenalidomide- or bortezomib- resistant | Bortezomib- resistant | Bortezomib- resistant | previous resistance to carfilzomib, or refractory to anti-CD38 | previous treatment with carfilzomib, or refractory to anti-CD38 | Bortezomib- resistant | Bortezomib- resistant | ||||||||||
| ITT population | 283 | 286 | 396 | 396 | 321 | 325 | 360 | 362 | 464 | 465 | 251 | 247 | 312 | 154 | 179 | 123 | 195 | 207 | 194 | 97 |
| PFS | 46 | 17.5 | 26.3 | 17.6 | 19.4 | 14.9 | 20.6 | 14.7 | 18.7 | 9.4 | 16.7 | 7.1 | NE | 15.8 | NE | 19.1 | 13.9 | 9.4 | 22.4 | 11.5 |
| HR (95% CI) | 0.42 (0.33-0.52) | 0.69 (0.57-0.83) | 0.71 (0.59-0.86) | 0.74 (0.59-0.94) | 0.53 (0.44-0.65) | 0.31 (0.25-0.39) | 0.63 (0.46-0.85) | 0.53 (0.32-0.89) | 0.70 (0.53-0.93) | 0.63 (0.44-0.90) | ||||||||||
| 1 prior line | 149 | 146 | 184 | 157 | 151 | 159 | 212 | 213 | 231 | 229 | 247 | 251 | 144 | 70 | 80 | 55 | 99 | 99 | 91 | 44 |
| PFS | 53.3 | 19.6 | 29.6 | 17.6 | NA | NA | 20.6 | 16.6 | 22.2 | 10.1 | 27 | 7.9 | NA | NA | 22.4 | 11.4 | ||||
| HR | 0.42 (0.30-0.57) | 0.713 (0.532-0.957 | 0.77 (0.59-1.01) | 0.882 (0.65-1.197) | 0.447 (0.330-0.606) | 0.22 (0.15-0.32) | 0.68 (0.40-1.14) | 0.589 (0.309-1.123) | 0.63 (0.41-0.96) | 0.75 (0.45-1.26) | ||||||||||
| 2-3 prior lines | 123 | 118 | 212 | 239 | 170 | 166 | 148 | 149 | 232 | 233 | 106 | 107 | 179 | 87 | 99 | 68 | 96 | 108 | 103 | 53 |
| PFS | NA | NA | 25.8 | 16.7 | NA | NA | NE | 12.9 | 14.9 | 8.4 | NA | NA | NA | NA | NA | NA | NA | NA | NE | 14 |
| HR (95% CI) | 2 prior lines: 0.39 (0.26-0.58) | 0.720 (0.561-0.923) | 0.68 (0.53-0.87) | 0.58 (0.401-0.838) | 0.604 (0.466-0.783) | 2 prior lines: 0.46 (0.30-0.72) | 0.61 (0.42-0.88) | 0.479 (0.294-0.778) | 2 prior lines: 0.65 (0.40-1.07) | 0.54 (0.33-0.88) | ||||||||||
| 3 prior lines: 0.48 (0.25-0.94) | 3 prior lines: 0.60 (0.33-1.07) | |||||||||||||||||||
The results according to the number of prior lines of therapy are also reported where available.
NA, not available; NE, not estimable/not reached.
Most patients who received a fixed duration bortezomib-based therapy at first line may still be eligible for a bortezomib- or PI-based regimen at relapse, provided that >6 months passed since the last dose of bortezomib to relapse/progression. PI-based regimens include Kd56,26 Dara-Vd,18 and anti-CD38 plus Kd56 (Dara-Kd5619 and Isa-Kd5620) (Table 2; Figures 1 and 2). SVd28 and venetoclax plus Vd (Ven-Vd)30 were also evaluated in bortezomib-sensitive patients with relapsed/refractory multiple myeloma (RRMM) with 1 to 3 prior lines. SVd28 prolonged median PFS (13.93 months vs 9.46 months for Vd; HR, 0.70), but in patients with prior exposure to bortezomib (n = 279), the HR was 0.81, and for those with prior exposure to carfilzomib (n = 41), HR was 0.62. In phase 3 BELLINI trial,30 just 10% of patients in the Ven-Vd and 15% in the Vd arm had t(11;14), and 78% and 81% had high BCL-2 expression defined by immunohistochemistry, respectively. Ven-Vd improved median PFS, driven by PFS benefit in t(11;14) group (HR, 0.11) and those with BCL2high expression (HR, 0.24); however, there was a signal of increased mortality in the Ven-Vd group. While median OS was similar between treatment groups for patients with t(11;14) or BCL2high, increased risk of death was observed among t(11;14)-negative and BCL2low patients.65 SVd and Ven-Vd, are not primary options for patients with relapse after 1 line of therapy given the available options.
Given the number of options, the choice may be significantly affected by the patient’s preferences, costs, specific patient and disease characteristics, as well as considerations regarding toxicity (Figure 2). For the above patient, Dara-Rd is our primary choice. Given the presence of t(4;14), a combination containing a PI with Rd (KRd, IRd) or a PI with monoclonal antibody (Dara-Kd, Isa-Kd), both of which are much more expensive and logistically challenging, may be alternative options. The presence of residual neuropathy is of concern for the use of Dara-Vd. Other combinations could include a doublet (Rd) or preferably a triplet such as bortezomib with lenalidomide and dexamethasone (VRd),66-69 VCd68 (provided there was no significant neuropathy), lenalidomide with cyclophosphamide and dexamethasone (RCd), or bendamustine-containing regimens.51,70-75
Clinical case 4
G.H., a 75-year-old man, was diagnosed with IgAκ myeloma, ISS-3, with extensive bone disease and moderate renal dysfunction. FISH studies revealed del17p in 50% of the cells. He started daratumumab with VMP, achieved a complete response (CR) after 8 cycles but remained minimal residual disease (MRD)-positive. During induction, he was hospitalized twice for pulmonary infections. At 22 months since diagnosis and while on maintenance with daratumumab, his monoclonal protein reappeared, and CT imaging revealed a paraosseous mass in L4 and L5.
Treatment of first relapse in daratumumab-refractory patients
Daratumumab-containing combinations have been approved for the primary therapy of patients who are eligible (Dara-VTd76) or ineligible (Dara-Rd77 and Dara-VMP78) for ASCT; additional combinations containing anti-CD38 (Dara-VRd, Isa-VRd, Isa-KRd) are under evaluation. In the event of progression while on such therapies, the disease may be refractory to daratumumab or daratumumab plus lenalidomide (Figure 1); in a few patients, progression while on Dara-VMP78 or Dara-VTD76 may also occur. The mechanisms of resistance to daratumumab are not fully understood.79-81 Many questions remain: is daratumumab (or anti-CD38) resistance reversible, and when? Can anti-CD38 therapy remain as part of the next treatment line? Are there any combinations of agents that can reverse anti-CD38 resistance?
There is limited data on the outcomes of patients relapsing after first-line daratumumab-based therapy. For patients not exposed to lenalidomide (such as those treated with Dara-VMP or Dara-VTD+ASCT), a combination containing lenalidomide plus a proteasome inhibitor (KRd53 or IRd55 or VRd) or a different class of monoclonal antibody such as elotuzumab (Elo-Rd)56 are reasonable options. Among doublets, Kd26 is an option, but efficacy in the setting of daratumumab resistance is unknown. For patients progressing on Dara-Rd (being refractory to both lenalidomide and daratumumab), proteasome inhibitor-based combinations such as Kd and Pom-Vd are reasonable options, but little is known for their activity in this setting. SVd is another option (but only 6% of patients had prior daratumumab in the BOSTON study), based on the different mechanism of activity of selinexor (the HR for PFS in the small subset of daratumumab refractory patients [n = 17] was 0.49 vs Vd). Other combinations such as carfilzomib, pomalidomide dexamethasone (KPd),38-40 or carfilzomib, cyclophosphamide, and dexamethasone (KCyd)19 can be considered; VCd or VMP may also be options. A non-PI-containing regimen with monoclonal antibody is Elo-Pd, although very few patients had prior daratumumab in the ELOQUENT-3 study.25
Belantamab mafodotin is an antibody-drug conjugate targeting B-cell maturation antigen (BCMA), which showed activity in a phase 2 study in heavily pretreated patients, of which 96% had prior exposure and resistance to daratumumab.82 Belantamab has been approved for single-agent use in patients with RRMM who have received at least 4 prior lines and disease refractory to at least 1 PI, an IMiD and an anti-CD38 monoclonal antibody, and combinations with PIs and IMiDs at earlier lines of therapy are under evaluation. Neither belantamab nor belantamab-based combinations have been approved for early lines, but in the setting of increasing daratumumab and lenalidomide resistance, they may soon become an option.
Although there is some evidence that daratumumab retreatment or continuing daratumumab as part of the next line of therapy can be efficacious in some patients,83,84 there is no data for daratumumab retreatment at second line. In a study evaluating the role of isatuximab in 32 heavily pretreated and daratumumab-refractory patients, the overall response rate (ORR) was low (1 patient had MR and 11 [34.5%] had stable disease for at least 8 weeks).85
For G.H., a lenalidomide and PI-based triplet, such as KRd or IRd, also considering the presence of high-risk cytogenetics, was our primary choice. Both regimens have been shown to improve outcomes in patients with such characteristics55,86 over Rd alone. Kd56 is a lenalidomide-free option (but with limited data in the setting of daratumumab refractoriness), perhaps with the addition of cyclophosphamide (KCyd).49,50 Alternative options may include VRd or Rd with cyclophosphamide. The history of frequent infections, moderate renal dysfunction, and the patient’s poor performance status are critical parameters for the choice of therapy (Figure 2). The patient and his family also wished to avoid frequent hospital visits, and IRd was prescribed. He achieved a partial response after 3 cycles, and without significant toxicity, he continued therapy for a total of 9 months, developing progressive disease with new bone lesions, anemia, and proteinuria.
Clinical case 5
I.J., a 55-year-old woman, was diagnosed with κ-light chain myeloma, R-ISS-2 (ISS-3 without high-risk cytogenetics, but presence of amp1q21). At diagnosis, she had amenia and multiple lytic lesions. After 4 cycles of VRd, she achieved a VGPR, stem cell collection yield was high, and cells were also stored for a potential second transplant. She received a single ASCT followed by 2 additional cycles of VRd and continued maintenance with lenalidomide achieving CR but remaining MRD-positive. Twenty-eight months after transplant, she developed disease progression with increasing free light chains and proteinuria, remaining asymptomatic.
Is there a role for salvage ASCT?
Salvage ASCT is an option for eligible patients who deferred ASCT at the time of first remission and may be an option for patients who relapse late after front-line ASCT. Two prospective studies have evaluated salvage ASCT. In the MRC study, bortezomib‐based reinduction was followed by either salvage ASCT or cyclophosphamide,87 and in the German Myeloma Multicenter Group (GMMG) study, patients with 1 to 3 prior lines received Rd reinduction (3 cycles) and were randomized to either ASCT (n = 139) and lenalidomide maintenance or continuous Rd (n = 138).88 Both studies are outdated given the new options; also, most patients receive lenalidomide maintenance after transplant. Most experts89 consider a second salvage ASCT for selected patients who, while on lenalidomide maintenance, have a progression-free interval after first transplant at least close or above the median expected PFS (about 3 to 4 years in most studies90); however, this approach may require reevaluation in view of the recent treatment advances.
For patients who are eligible but have not received ASCT at first remission, a salvage transplantation should be strongly considered. In the IFM2009 and EMN02 studies, the use of salvage transplant in patients that did not receive it at first remission resulted in similar OS between those that received ASCT at first remission or relapse.91,92 An open issue remains optimal reinduction. A nonlenalidomide-containing regimen that includes a proteasome inhibitor is reasonable in those receiving lenalidomide maintenance (EMN011 study used KPd reinduction40); however, in the case of a biochemical-only relapse, moving directly to high dose melphalan may be an option. For the patient above (I.J.), salvage transplantation was discussed along with other available options. The 28-month period that elapsed since ASCT was felt shorter than the expected 3 to 4 years of relapse-free interval. The initial decision was to manage the patient without ASCT taking into consideration lenalidomide refractoriness; however, the potential use of high-dose melphalan at a later point, given the availability of a graft, remained an option.
Multirefractory patients
Treatment of patients who have received ≥2 lines of therapy is challenging,93 especially among those that have been exposed to all major drug classes (as with our patients G.H. and I.J). More often, patients have been exposed to 1 drug from each class, but patients who are refractory to 2 PIs, 2 immunomodulatory drugs (IMiDs), and a CD38 mAb (penta-refractory) have a median OS of just 5.6 months.94
For patients who have been exposed or are refractory to both bortezomib and lenalidomide and have not received an anti-CD38 MoAb, a CD38-based regimen is a primary option (data are available for Isa-Pd,24 Dara-Pd,23 or Elo-Pd25 in this setting). For anti-CD38 plus PI combinations such as Dara-Kd19 or Isa-Kd,20 the analysis of double refractory subgroups is not available (but these include small numbers), with results being better in bortezomib-exposed but sensitive patients (Table 2; Figures 1 and 2). Another option not evaluated in phase 3 studies includes various combinations of Pd with cyclophosphamide.32-37 Other options for patients who remain nonrefractory to bortezomib include PVd,95 SVd,28 and for those with t(11;14), Ven-Vd30 (but has not been approved).
For patients who are refractory to at least a PI, an IMiD and anti-CD38, selinexor-dexamethasone (Sd) and belantamab mafodotin monotherapy are options. Selinexor is an XPO1 inhibitor; in the phase 2b STORM study, median PFS with oral Sd (n = 122 triple-class refractory patients, median of 7 prior lines) was 3.7 months, and median OS was 8.6 months.96 In the phase 2 DREAM-2 trial, 196 patients with triple-class refractory disease (96% were daratumumab-exposed) received belantamab mafodotin monotherapy, and median PFS was 2.9 and 4.9, respectively, for 2.5 and 3.4 mg/kg doses (not different).82 In the 13-month follow-up of the study, the median duration of response at the 2.5 mg/kg dose was 11 months, and median OS was 14.9 months (53% OS for both dose levels at 12 months).97 However, ocular toxicity/keratopathy was common and, although reversible, resulted in treatment discontinuation in 2% of patients, dose reduction in 27% of patients, and more often in dose delays.
A new generation of IMiDs (or cereblon modulators) such as iberdomide show activity in heavily pretreated patients, manageable toxicity, and are explored in combinations with several other agents (PIs, anti-CD38 monoclonal antibodies). However, their regulatory approval requires substantially more data.98
New immunotherapy options include cellular therapies with chimeric antigen receptor T cells (CAR-T cells) targeting BCMA (or other antigens) and bispecific T-cell engaging monoclonal antibodies. The CAR-T product idecaptagene cicleucel (ide-cel) has been evaluated in a phase 1 and a phase 2 study. In the phase 1 study (n = 33 patients with advanced disease but not penta-refractory), the response rate was 85% (45% CR, all MRD-negative), and median PFS was 11.8 months.99 In the phase 2 study, KarMMa, 128 of 140 enrolled patients received ide-cel target doses (150 × 106 − 450 × 106 CAR T-cells). After a median follow-up of 13.3 months, overall response was 73%, 33% achieved CR (26% were MRD-negative), and median PFS was 8.8 months.100 Ciltacabtagene autoleucel (cilta-cel) is based on biepitopic BCMA targeting.101 In the phase 1b/2 CARTITUDE-1 study, patients with 3 or more prior lines or refractory to a PI and an IMiD and anti-CD38 (n = 97, median 6 prior lines) received a single cilta-cel infusion. The ORR was 97% with 67% sCRs and a 12-month PFS rate of 77% and OS of 89%.102 Other studies evaluate alternative CAR-T cell constructs and different strategies, different manufacturing approaches,103-105 dual targeting,106 and allogeneic CAR-T cells.107 CAR T-cell therapies targeting other molecules such as SLAMF7, CD38, NKG2D (KLRK1) ligands, or CD138 are under evaluation.108 Cellular therapies are probably the most active therapy in the setting of advanced disease, but major concerns include limited availability, financial costs, progression during cell product manufacturing, development of resistance, and potential crossresistance with other anti-BCMA targeting therapies. The FDA has approved ide-cel for patients that have received at least 4 prior lines and EMA for patients who have received at ≥3 previous therapies, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody.
Several bispecifics are in development and presented phase 1 results (Table 3). Teclistamab is a bispecific that binds BCMA and CD3 to redirect T cells to myeloma cells. In a phase 1 study, teclistamab was administered by IV or subcutaneously in 157 patients (median 6 prior lines) that received at least 1 dose once per week; subcutaneous administration was identified as the recommended phase 2 dose. The ORR in response-evaluable patients treated at the active doses (n = 86) was 67% (63% VGPR).109 Bispecifics targeting antigens beyond BCMA,110-113 such as GPRC5D (GPRC5DxCD3, talquetamab)114 and FcRH5 (FcRH5xCD3, cevostamab)115 are in clinical development. Initial dose-escalation studies have shown high response rates (up to 66%) in patients with multirefractory disease, especially at the higher dose levels. However, cytokine release syndrome and neurotoxicity are also observed with bispecifics, and studies are ongoing to optimize their administration and evaluate their position in myeloma therapy. Given their immediate availability, in contrast to CAR-T-cell products, T-cell engager antibodies could be a more attractive immunotherapy option, but, as of today, are only available in the setting of clinical trials.
New bispecific monoclonal antibodies under clinical development for patients with RR myeloma
| Bispecific antibody . | Antibody structure . | Target . |
|---|---|---|
| AMG 420 | BiTE | BCMA × CD3 |
| AMG 701 | Extended half-life, scFv plus Fc region | BCMA × CD3 |
| PF-0686135 (elranatamab) | Full-length, humanized IgG2a | BCMA × CD3 |
| REGN5458 | Fc Fab arms | BCMA × CD3 |
| Teclistamab | humanized, IgG Fc | BCMA × CD3 |
| CC-93269 | 2-arm humanized IgG1 Fc, binds bivalently to BCMA and monovalently to CD3 in a 2 + 1 format | BCMA × CD3 |
| TNB-383B | IgG4 Fc. anti-CD3 moiety preferentially activates effector over Tregs; 2 heavy chain-only anti-BCMA moieties | BCMA × CD3 |
| BFCR4350A | Humanized IgG1 Fc | FcRL5 × CD3 |
| Talquetamab | IgG4 Fc | GPRC5D × CD3 |
| Bispecific antibody . | Antibody structure . | Target . |
|---|---|---|
| AMG 420 | BiTE | BCMA × CD3 |
| AMG 701 | Extended half-life, scFv plus Fc region | BCMA × CD3 |
| PF-0686135 (elranatamab) | Full-length, humanized IgG2a | BCMA × CD3 |
| REGN5458 | Fc Fab arms | BCMA × CD3 |
| Teclistamab | humanized, IgG Fc | BCMA × CD3 |
| CC-93269 | 2-arm humanized IgG1 Fc, binds bivalently to BCMA and monovalently to CD3 in a 2 + 1 format | BCMA × CD3 |
| TNB-383B | IgG4 Fc. anti-CD3 moiety preferentially activates effector over Tregs; 2 heavy chain-only anti-BCMA moieties | BCMA × CD3 |
| BFCR4350A | Humanized IgG1 Fc | FcRL5 × CD3 |
| Talquetamab | IgG4 Fc | GPRC5D × CD3 |
Authorship
Contribution: E.K., E.T., and M.A.D. reviewed the literature; E.K. drafted the first draft of the manuscript; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: E.K. provided consultancy, received honoraria, travel/accommodations/expenses, and research funding from Janssen; provided consultancy for Pfizer; provided consultancy, received honoraria and travel/accommodations/expenses from Genesis Pharma; provided consultancy, received honoraria and research funding from Amgen; provided consultancy, received honoraria and travel/accommodations/expenses from Takeda. E.T. received honoraria, travel expenses, and research funding from Janssen; received honoraria, travel expenses, and research funding from Takeda; received honoraria from Celgene; received honraria from Medison; received honoraria and research funding from Amgen; received honoraria, travel expenses, and research funding from Genesis. M.A.D. provided consultancy, membership on an entity's board of directors or advisory committees, and received personal fees from BMS; provided consultancy, received honoraria, membership on an entity's board of directors or advisory committees, received personal fees, and was member of speakers bureau for Celgene; provided consultancy, received honoraria, membership on an entity’s board of directors or advisory committees, received personal fees, research funding, and member of speakers bureau for Takeda; provided consultancy, received honoraria, membership on an entity's board of directors or advisory committees, received personal fees and research funding, and member of speakers bureau for Janssen; and provided consultancy, received honoraria, membership on an entity's board of directors or advisory committees, received personal fees, research funding, and member of speakers bureau for Amgen.
Correspondence: Meletios A. Dimopoulos, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, 80 Vas. Sofias Ave & Lourou Str, 11528, Athens, Greece; e-mail: mdimop@med.uoa.gr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal