Key Points
Zinc released by thymocytes after HCT conditioning is sensed by GPR39 and mediates epithelial repair.
Pharmacologic stimulation of GPR39 promotes thymic function and T-cell reconstitution after HCT.
Abstract
Prolonged lymphopenia represents a major clinical problem after cytoreductive therapies such as chemotherapy and the conditioning required for hematopoietic stem cell transplant (HCT), contributing to the risk of infections and malignant relapse. Restoration of T-cell immunity depends on tissue regeneration in the thymus, the primary site of T-cell development, although the capacity of the thymus to repair itself diminishes over its lifespan. However, although boosting thymic function and T-cell reconstitution is of considerable clinical importance, there are currently no approved therapies for treating lymphopenia. Here we found that zinc (Zn) is critically important for both normal T-cell development and repair after acute damage. Accumulated Zn in thymocytes during development was released into the extracellular milieu after HCT conditioning, where it triggered regeneration by stimulating endothelial cell production of BMP4 via the cell surface receptor GPR39. Dietary supplementation of Zn was sufficient to promote thymic function in a mouse model of allogeneic HCT, including enhancing the number of recent thymic emigrants in circulation although direct targeting of GPR39 with a small molecule agonist enhanced thymic function without the need for prior Zn accumulation in thymocytes. Together, these findings not only define an important pathway underlying tissue regeneration but also offer an innovative preclinical approach to treat lymphopenia in HCT recipients.
Introduction
The thymus, which is the primary site of T-cell generation, is extremely sensitive to insult but also has a remarkable capacity for endogenous repair.1,2 However, although there is likely continual thymic involution and regeneration in response to everyday insults such as stress and infection, profound thymic damage such as that caused by common cancer therapies and the conditioning regimens used as part of hematopoietic stem cell transplantation (HCT) contributes to prolonged T-cell depletion. Posttransplant lymphopenia precipitates high morbidity and mortality from opportunistic infections and likely facilitates malignant relapse.3-9 Currently, there are no approved therapies to enhance posttransplant T-cell reconstitution in recipients of HCT.
Zinc (Zn) is the second most abundant trace element in the body, capable of interacting with >300 proteins involved in almost all aspects of cell function,10-12 including a well-established role in immune health.13-15 Much of what we know about the effect of Zn on immune function comes from studies in which dietary Zn has been deficient, either due to reduced intake caused by malnourishment or via genetic means such as loss of function of ZIP4, a Zn transporter, which clinically leads to the condition Acrodermatitis enteropathica.16-19 In these settings of Zn deficiency (ZD), widespread immune effects can be seen, including defective B-cell development, atrophy of the thymus, and disrupted T-cell function.13,20-24 However, while ZD is known to lead to thymic involution and supplementation with dietary Zn can ameliorate this phenotype,15,25 the mechanisms by which Zn acts on thymic function are poorly understood.
Here, we have shown that not only is Zn important for the differentiation and development of thymocytes during T-cell development but also its translocation after acute injury such as that caused by total body irradiation (TBI) can directly stimulate the production of BMP4 by endothelial cells (ECs), which has recently been found to be a critical pathway for endogenous thymic regeneration after acute injury.26 This putative role for Zn as a damage-associated molecular pattern was mediated by signaling through the cell surface Zn receptor GPR39. Notably, whereas dietary ZS enhanced T-cell reconstitution after allogeneic HCT, direct pharmacologic stimulation of GPR39 enhanced thymic regeneration and abrogated the need for a complex and prolonged Zn administration. Therefore, the studies outlined here have the potential to define an important pathway underlying tissue regeneration and offer an innovative clinical approach to enhance T-cell reconstitution in recipients of HCT.
Methods
Mice
Four- to 6-week-old male or female C57BL/6 (CD45.2) or B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice were obtained from Jackson Laboratories (Bar Harbor, ME). RAG2-GFP mice were kindly provided by Dr. Pamela Fink.27 Custom-made diets (1 ppm of Zn compared with 35 ppm of control diet) were purchased from Labdiet (St. Louis, MO). Zn supplementation (ZS) was administered orally by dissolving ultrapure Zn sulfate monohydrate (Alfa Aesar, Haverhill, MA) in drinking water (1.06 g/mL, which delivered 300 mg/kg per mouse per day based on average water consumption).
Sublethal TBI was given at a dose of 550 cGy with no hematopoietic rescue. HCT mice received 5 to 10 × 106 T-cell depleted bone marrow (BM) cells/recipient. B6 HCT recipients received 1100 cGy TBI (2 × 550 cGy); BALB.b recipients received 900 cGy (2 × 450 cGy). Graft-versus-host disease (GVHD) was induced with 2 × 106 T cells/recipient.28 T-cell depletion was performed with CD3 beads (Miltenyi Biotech, Bergisch Gladbach, Germany, #130-094-973). All TBI experiments were performed with a 137Cs γ-radiation source.
TC-G 1008 (Tocris, Bristol, UK) at the dose of 20 mg/kg per mouse was given via micropipette-guided administration29 daily from day 0 to day 8 and then biweekly. Dorsomorphin dihydrochloride (12.5 mg/kg per day) was given 1 day before TBI and then twice daily from day 1. Mice were maintained at Fred Hutchinson Cancer Research Center (Seattle, WA). Animals were allowed to acclimatize for at least 2 days before experimentation, which was performed according to Institutional Animal Care and Use Committee guidelines.
Cell isolation
Individual or pooled single-cell suspensions from thymus or spleen were obtained as previously described.26,30,31 Axillary and inguinal lymph nodes were collected and mechanically dissected before counting the cells and staining them with flow cytometry antibodies. Cell counts were performed by Z2 particle counter (Beckman Coulter, Pasadena, CA), Spark 10M (Tecan, Zürich, Switzerland), or hemocytometer. CD45− cells were enriched by magnetic bead separation using LS columns and CD45 beads (Miltenyi Biotech,). Peripheral blood was collected into EDTA capillary pipettes (Drummond Scientific, Broomall, PA). Peripheral blood counts were performed on Element Ht5 automatic counter (Heska, Loveland, CO).
Cell cultures
exECs were generated as previously described.32 Cells were cultured in presence of ultrapure Zn sulfate monohydrate purchased from Alfa Aesar (Haverhill, MA, #1113809) or sodium pyrythione (Sigma Aldrich, St. Louis, MO, #H3261-1G). TC-G1008 was used in cell cultures at a final concentration of 25 μM. Silencing of the zinc receptor GPR39 was performed by electroporation using Nucleofector electroporation kit (VPI-1001, Lonza; Program M-003, Nucleofector 2b, Lonza), and the GPR39 siRNA Silencer Select (Thermo Fisher Scientific, Waltham, MA). Mouse C9 (cTEC) and TE-71 (mTEC) cells were kindly provided by A. Farr, University of Washington.
ELISA and western blot
Cell culture or tissue lysates were prepared in RIPA buffer (Thermo Fisher Scientific) as previously described26 and normalized by BCA assay (Thermo Fisher Scientific). BMP4 levels were quantified by enzyme-linked immunosorbent assay (ELISA) (LSBio, Seattle, WA) and read on a Spark 10M plate reader (Tecan). Cortisol levels were measured with ELISA on peripheral blood (R&D Systems, Minneapolis, MN). Proteins were resolved on 12% SDS-PAGE and transferred onto polyvinylidene difluoride(PVDF) membranes (Bio-Rad, Hercules, CA). Blots were analyzed using the enhanced chemiluminescence detection system or scanned with an Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE). In vitro cell proliferation was measured using the CellTiter Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI).
Flow cytometry, EdU incorporation, flow cytometry sorting, and multidimensional analyses
For flow cytometry and cell sorting, surface antibodies against CD45 (30-F11), CD31 (390 or MEC13.3), CD90.2 (30-H12), TER-119 (TER-119), CD4 (RM4-5 or GK1.5), CD8 (53-6.7), TCRβ (H57-597), CD3 (145-2C11), CD44 (IM7), CD25 (PC 61.5), CD62L (MEL14), MHC-II IA/IE (M5/114.15.2), EpCAM (G8.8), Ly51 (6C3), CD11c (HL3), IL-7Rα (A7R34), CCR6 (140706), CD45.1 (A20), CD45.2 (104), ki-67 (16A8), and PDGFRα (APA5) were purchased from BD Biosciences (Franklin Lakes, NJ), BioLegend (San Diego, CA), or eBioscience (San Diego, CA). Ulex europaeus agglutinin 1 (UEA-1), conjugated to fluorescein isothiocyanate (FITC) or Biotin, was purchased from Vector Laboratories (Burlingame, CA). GPR39 conjugated to FITC was purchased from Signalway Antibody (College Park, MD). Red blood cell lysis was performed with red cell lysis (ACK) buffer (Thermo Fisher Scientific). Flow cytometry was performed on a Fortessa X50 (BD Biosciences), and cells were sorted on an Aria II (BD Biosciences) using FACSDiva (BD Biosciences). For intracellular cytokine analysis, cells were fixed and permeabilized using Fix Buffer I and Phospho-Perm Buffer III from BD Bioscience. Fluozin-3 am was purchased from Thermo Fisher. Analysis was performed by FlowJo (Treestar Software, Ashland, OR). EdU (5-ethynyl-2′-deoxyuridine, Thermo Fisher Scientific) was administered to mice intraperitoneally (100 µL at a concentration of 2 mg/mL) 4 hours before tissue was harvested and detection performed per manufacturer's instructions. For UMAP (uniform manifold approximation and projection) analysis, gated CD45+ T cells were exported in R (version 4.0.2) for further analyses using a custom-made script based on Nowicka et al.34
PCR and microarray
Reverse transcription polymerase chain reaction (PCR) was performed with iScript Clear gDNA cDNA synthesis kit (Bio-Rad) on CFX96 (Bio-Rad) with iTaq Universal SYBR Green (Bio-Rad). Relative amounts of mRNA were calculated by the comparative ΔC(t) method or as relative expression. SYBR Green gene expression assays for qPCR, including Bmp4 (Mm00432087_m1), Foxn1 (Mm00433948_m1), β-actin, Gpr39, and Top1 were all purchased from Life Technologies (Carlsbad, CA) and Bio-Rad (b-actin: synthesized by IDT, F: 5′-CACTGTCGAGTCGCGTCC-3′, R: 5′- TCATCCATGGCGAACTGGTG-3′; Top1: synthesized by IDT, sequences provided by PrimerBank, PrimerBank ID 6678399a1; Bmp4: Biorad qMmuCED0046239; Foxn1: Biorad qMmuCED0044924). Microarray data from CD45− cells was analyzed on days 0, 4, and 7 as previously described26 (GSE106982) and differential gene expression of GPR39 was calculated.
ICP-MS
Whole thymus tissue was harvested, weighed, and immediately frozen; thymocytes were collected by mechanical dissociation and the remnant stromal component was discarded. Each sample was added to trace meal clean plastic vials that had been previously acid leached and rinsed several times with high-purity 18-MOhm water. Samples were digested in 1:1 vol/vol mix of 50% HNO3 and 10% H2O2 in a plastic and trace metal clean laminar fume hood. Samples were analyzed on an iCAP RQ (Thermo Fisher) inductively coupled plasma mass spectrometry (ICP-MS) (University of Washington, TraceLab) in 2% optima grade nitric acid. Three isotopes of Zn were analyzed and cross referenced for isobaric interferences, and Zn-66 was chosen because it exhibited the best signal with the least interference. Precise mass of the initial sample, final sample, and each aliquot was taken into account to calculate concentration of Zn (milligrams of Zn per gram of thymic tissue or milligrams of Zn per gram of supernatant). A rhodium internal standard was used to correct for changes in plasma ionization efficiency, and external standards, including USGS T231, were used to ensure accuracy and traceability.
pERK1/2 imaging
Thymuses were snap-frozen and mounted in optimal cutting temperature compound (OCT, TissueTek), and 10-µm sections were fixed in paraformaldehyde. Sections were stained with anti-ERK1/2 (Invitrogen, Waltham, MA), anti-PECAM (R&D Systems), and detected with Alexa Fluor 488 or 568 (Thermo Fisher Scientific). 4′,6-diamidino-2-phenylindole (DAPI) was stained at a concentration of 300 nM, and sections were mounted with Vectashield Plus (Vecto Laboratories, Burlingame, CA) and imaged using a TissueFAX Plus (Tissuegnostic, Tarzana, CA). Fluorescent images of whole thymic tissue were analyzed using ImageJ 1.5i. Regions of interest were selected using 4′,6-diamidino-2-phenylindole (DAPI), and fluorescence measurements were taken on green fluorescent protein (GFP) (signal of pERK1/2) image. Multiple sections of each thymus were analyzed and averaged.
Statistics
Statistical analysis between 2 groups was performed with the nonparametric, unpaired Mann-Whitney U test. Statistical comparison among 3 or more groups was performed with the nonparametric, unpaired Kruskall-Wallis test. All statistics were calculated and display graphs were generated in Graphpad Prism.
Results
Zn is crucial for steady-state T-cell development and promoting regeneration after acute damage
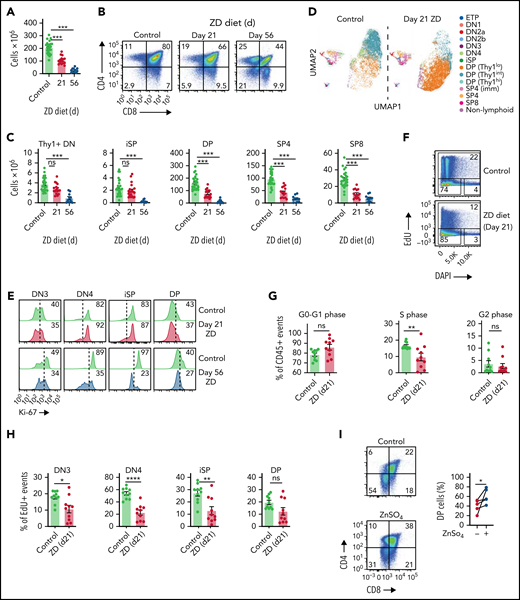
Modeling the effect of Zn on baseline thymic function, mice fed a Zn-deficient diet exhibited reduced thymic cellularity (Figure 1A; supplemental Figure 1A, available on the Blood Web site) in as little as 3 weeks of ZD treatment when compared with age-matched mice that received normal chow. These effects were observed even when there were no gross phenotypes such as weight loss (supplemental Figure 1B). Although Zn has previously been shown to affect peripheral T cells,13,21,35,36 there was no effect of the ZD treatment on absolute lymphocyte count at 21 days (supplemental Figure 1C), with significant decrease of naïve T cells seen from 5 weeks of ZD diet (supplemental Figure 1D). Importantly, levels of cortisol, a stress hormone with considerable negative effects on the thymus,2 were consistent throughout the experiment (supplemental Figure 1E). Cell depletion was not uniform among developing T cells because at day 21 there was a significant decrease only in double positive (DP) and single positive (SP) CD4+ and CD8+ (SP4 and SP8) cells but no change in the earlier double negative (DN), early thymic progenitors, or intermediate SP (iSP) thymocytes (Figure 1B-C; supplemental Figure 1F). Within DP thymocytes, there appeared to be a block after ZD treatment that was marked by different level of expression of Thy1 (Figure 1D). Although moderately decreased proliferation was observed by Ki67 expression after 3 weeks of ZD, all thymocyte subsets had considerable loss of proliferation by 8 weeks (Figure 1E; supplemental Figure 1G), by EdU incorporation we observed decreased S phase as early as 21 days (Figure 1F-G), with decreased proliferation in the thymocyte stages preceding DP (Figure 1H). These findings are consistent with a role for Zn in DNA synthesis.37 To confirm the importance of Zn on T-cell maturation, we cocultured lineage-negative BM isolated from C57BL/6 mice on the OP9-DLL1 system, which is able to support T-cell development in vitro.33,38 Compared with control, BM cultured in media with ZN sulfate (ZnSO4) showed more robust production of DP (Figure 1I).
Dietary deficiency of Zn rapidly impairs T-cell development. Six- to 8-week-old female C57BL/6 mice were fed a normal or Zn-deficient diet for up to 8 weeks. (A) Total thymus cellularity from untreated mice or after 21 or 56 days of ZD (untreated, n = 24, combined from animals harvested alongside either day 21 or day 56 mice; day 21, n = 15 over 3 independent experiments; day 56, n = 10 over 2 independent experiments). (B) Concatenated flow cytometry plots displaying CD4 and CD8 expression in the thymus (plots were gated on viable CD45+ cells). (C) Total number of CD4−CD8−Thy1+ DN, CD3−CD4−CD8+ iSP, CD4+CD8+ DP, CD3+CD8−CD4+ SP4, or CD3+CD8+ CD4− SP8 thymocytes from untreated mice or after 21 or 56 days of ZD (untreated, n = 24, combined from animals harvested alongside either day 21 or day 56 mice; day 21, n = 15 over 3 independent experiments; day 56, n = 10 over 2 independent experiments). (D) Multiparameter flow cytometry data from untreated mice or 21 days after ZD was placed in UMAP space and clusters were generated based on relative MFI of markers of thymocyte maturation (CD25, CD44, Thy1, CD4, CD8, CD3). (E) Concatenated flow cytometry plots showing Ki-67 expression in DN3, DN4, iSP, and DP thymocytes from untreated mice or after 21 or 56 days of ZD. (F) Concatenated flow cytometry plots showing EdU and DAPI expression in CD45+ events from untreated mice or after 21 days of ZD. (G) Proportion G0-G1, S, and G2 cells assessed with EdU/DAPI on CD45+ events from untreated mice or after 21 days of ZD (n = 10, combined from animals harvested over 3 independent experiments). (H) Proportion of EdU+ events (S phase) gated on CD4−CD8−Thy1+ CD25+CD44− DN (DN3), CD4−CD8−Thy1+ CD25-CD44− DN (DN4), CD3−CD4−CD8+ (iSP), CD4+CD8+ (DP) from untreated mice or after 21 days of ZD. (I) Lineage-depleted BM cells were isolated from untreated 6-week-old C57BL/6 mice and cocultured with OP9-DL1 cells for 30 days in the presence or absence of ZnSO4 (10 µM added from day 0). Concatenated flow cytometry plots displaying CD4 and CD8 expression at day 30 (n = 5 independent experiments). Graphs represent mean ± standard error of the mean (SEM); each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Dietary deficiency of Zn rapidly impairs T-cell development. Six- to 8-week-old female C57BL/6 mice were fed a normal or Zn-deficient diet for up to 8 weeks. (A) Total thymus cellularity from untreated mice or after 21 or 56 days of ZD (untreated, n = 24, combined from animals harvested alongside either day 21 or day 56 mice; day 21, n = 15 over 3 independent experiments; day 56, n = 10 over 2 independent experiments). (B) Concatenated flow cytometry plots displaying CD4 and CD8 expression in the thymus (plots were gated on viable CD45+ cells). (C) Total number of CD4−CD8−Thy1+ DN, CD3−CD4−CD8+ iSP, CD4+CD8+ DP, CD3+CD8−CD4+ SP4, or CD3+CD8+ CD4− SP8 thymocytes from untreated mice or after 21 or 56 days of ZD (untreated, n = 24, combined from animals harvested alongside either day 21 or day 56 mice; day 21, n = 15 over 3 independent experiments; day 56, n = 10 over 2 independent experiments). (D) Multiparameter flow cytometry data from untreated mice or 21 days after ZD was placed in UMAP space and clusters were generated based on relative MFI of markers of thymocyte maturation (CD25, CD44, Thy1, CD4, CD8, CD3). (E) Concatenated flow cytometry plots showing Ki-67 expression in DN3, DN4, iSP, and DP thymocytes from untreated mice or after 21 or 56 days of ZD. (F) Concatenated flow cytometry plots showing EdU and DAPI expression in CD45+ events from untreated mice or after 21 days of ZD. (G) Proportion G0-G1, S, and G2 cells assessed with EdU/DAPI on CD45+ events from untreated mice or after 21 days of ZD (n = 10, combined from animals harvested over 3 independent experiments). (H) Proportion of EdU+ events (S phase) gated on CD4−CD8−Thy1+ CD25+CD44− DN (DN3), CD4−CD8−Thy1+ CD25-CD44− DN (DN4), CD3−CD4−CD8+ (iSP), CD4+CD8+ (DP) from untreated mice or after 21 days of ZD. (I) Lineage-depleted BM cells were isolated from untreated 6-week-old C57BL/6 mice and cocultured with OP9-DL1 cells for 30 days in the presence or absence of ZnSO4 (10 µM added from day 0). Concatenated flow cytometry plots displaying CD4 and CD8 expression at day 30 (n = 5 independent experiments). Graphs represent mean ± standard error of the mean (SEM); each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Perhaps unsurprisingly given its importance for maintaining thymopoiesis, mice that had been on a ZD diet exhibited poorer regeneration after a sublethal dose of TBI (SL-TBI, 550 cGy) (Figure 2A), reflected among all developing thymocytes (supplemental Figure 2A) and supporting thymic epithelial cell (TEC) subsets (Figure 2B). The thymus is extremely sensitive to GVHD, even in situations in which GVHD may not be detected in classic target organs such as skin, gut, or liver.30,39,40 Surprisingly, ZD even worsened thymic reconstitution in a mouse model of acute GVHD in which significant damage separately occurred (Figure 2C; supplemental Figure 2B). Importantly, the effects of ZD on thymic repair in non-GVHD damage models could be ameliorated by supplementation of drinking water with Zn sulfate (300 mg/kg per day) beginning on the day of irradiation (Figure 2D). These findings demonstrate that even a short-term reduction in Zn intake has a detrimental impact on thymopoiesis and the transition from DN to DP stage of T-cell development as well as postdamage thymic reconstitution.
Dietary Zn intake dictates regenerative capacity of the thymus after damage. (A-B) Six- to 8-week-old female C57BL/6 mice were fed a normal or ZD diet for 21 days, at which point mice were given a sublethal dose of TBI (550 cGy). (A) Total thymic cellularity at days 7 and 14 after TBI (n = 10/treatment per timepoint across 2 independent experiments). (B) Total number of CD45−EpCAM+MHCII+UEA1loLy51hi (cTECs) and CD45−EpCAM+MHCII+UEA1hiLy51lo (mTECs) at days 7 and 14 after TBI (n = 10/treatment per timepoint across 2 independent experiments). (C) Six- to 12-week-old female BALB.B mice were fed with normal or ZD diet for 21 days, at which point mice were given a lethal dose of TBI (900 cGy) and 10 × 106 T-cell–depleted BM cells from 6- to 8-week-old C57BL/6 mice. One cohort also received 2 × 106 T cells to induce GVHD; thymic cellularity was quantified on day 14 after allo-HCT (n = 5-6/group). (D) Six- to 8-week-old female C57BL/6 mice were fed with normal or ZD diet for 21 days, at which point mice were given 550 cGy TBI and total thymus cellularity quantified on day 14. One cohort was given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) from day 0 until analysis on day 14 (n = 10/group across 2 independent experiments). (E-F) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day ZnSO4) for 21 days, at which point mice were given 550 cGy of TBI. Mice were maintained on ZnSO4 in drinking water and the thymus was analyzed on day 0 or 7 (day 0: untreated, n = 22; ZD, n = 10 across 2 independent experiments; day 7: untreated, n = 15; ZD, n = 15, across 3 independent experiments). (E) Total thymic cellularity. (F) Total number of cTECs and mTECs. (G) Total number of Ki-67+ TECs. Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Dietary Zn intake dictates regenerative capacity of the thymus after damage. (A-B) Six- to 8-week-old female C57BL/6 mice were fed a normal or ZD diet for 21 days, at which point mice were given a sublethal dose of TBI (550 cGy). (A) Total thymic cellularity at days 7 and 14 after TBI (n = 10/treatment per timepoint across 2 independent experiments). (B) Total number of CD45−EpCAM+MHCII+UEA1loLy51hi (cTECs) and CD45−EpCAM+MHCII+UEA1hiLy51lo (mTECs) at days 7 and 14 after TBI (n = 10/treatment per timepoint across 2 independent experiments). (C) Six- to 12-week-old female BALB.B mice were fed with normal or ZD diet for 21 days, at which point mice were given a lethal dose of TBI (900 cGy) and 10 × 106 T-cell–depleted BM cells from 6- to 8-week-old C57BL/6 mice. One cohort also received 2 × 106 T cells to induce GVHD; thymic cellularity was quantified on day 14 after allo-HCT (n = 5-6/group). (D) Six- to 8-week-old female C57BL/6 mice were fed with normal or ZD diet for 21 days, at which point mice were given 550 cGy TBI and total thymus cellularity quantified on day 14. One cohort was given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) from day 0 until analysis on day 14 (n = 10/group across 2 independent experiments). (E-F) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day ZnSO4) for 21 days, at which point mice were given 550 cGy of TBI. Mice were maintained on ZnSO4 in drinking water and the thymus was analyzed on day 0 or 7 (day 0: untreated, n = 22; ZD, n = 10 across 2 independent experiments; day 7: untreated, n = 15; ZD, n = 15, across 3 independent experiments). (E) Total thymic cellularity. (F) Total number of cTECs and mTECs. (G) Total number of Ki-67+ TECs. Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Because thymic function at both baseline and after damage was so sensitive to Zn availability, we hypothesized that dietary ZS could improve thymic reconstitution after acute insult. Mice were put on ZS (300 mg/kg per day per mouse of ZnSO4 monohydrate) in drinking water25 for 3 weeks prior to SL-TBI and maintained for 7 days after. Although we observed no difference in baseline thymic cellularity between control and ZS mice after 3 weeks of treatment, mice that received ZS treatment exhibited improved reconstitution after SL-TBI (Figure 2E), reflected by individual thymocyte populations (supplemental Figure 3) and within TEC subsets (Figure 2F). Both the absolute number and the proportion of proliferating TECs, measured by the expression of Ki-67, were higher in the thymuses from mice that received ZS (Figure 2G).
Zn stimulates the production of BMP4 by thymic ECs
Given the increased proliferation of TECs after ZS, we tested the direct effect of ZnSO4 on proliferation of mouse cortical (C9) and medullary (TE-71) TEC cell lines. We did not observe any direct effect of Zn on TEC proliferation or their ability to express key thymopoietic transcription factors such as FOXN1 (supplemental Figure 4A-B). One mechanism by which TECs are induced to proliferate is via BMP4 stimulation, which is produced by ECs in response to damage and can mediate thymic repair by stimulating TEC regeneration.26,41 Interestingly, a body of work demonstrates that Zn plays an important role in vascular integrity and EC response to stress.42-44
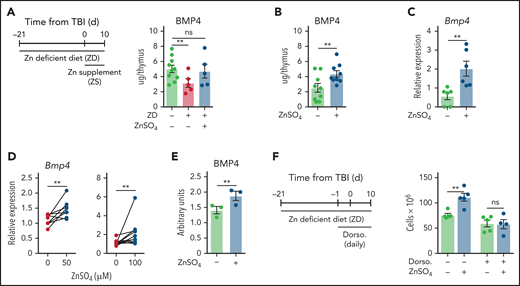
To determine if BMP4 could be mediating the effect of Zn in thymic regeneration, we first assessed the level of BMP4 in the thymus of mice that had received either a ZD diet for 3 weeks before TBI alone (and throughout the study) or mice that had received a ZD diet but had also been given ZS in drinking water. BMP4 was measured at day 10 after TBI, a timepoint at the peak of expression after damage.26 Using this approach, we found that mice that had received a ZD diet had significantly reduced levels of BMP4 in the thymus, but dietary supplementation of Zn restored BMP4 levels (Figure 3A). Consistent with these findings, mice that had been given ZS without prior ZD had increased levels of BMP4 at day 10 after TBI alone (Figure 3B), and purified ECs expressed significantly more Bmp4 as measured by qPCR (Figure 3C). Together, these findings suggest that Zn is involved not only in thymocyte maturation but also in overall thymic regeneration by stimulating the production of BMP4 from EC.
Zn stimulates the production BMP4 by ECs. (A) 6- to 8-week-old female C57BL/6 mice were fed a normal or ZD diet for 21 days, at which point mice were given 550 cGy TBI. One cohort was given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) from day 0. Levels of BMP4 were quantified by ELISA at day 10 after TBI (n = 5-10/group from 1 independent experiment). (B-C) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given 550 cGy TBI. Mice were maintained on Zn-supplemented drinking water for the duration of the study. (B) BMP4 levels measured by ELISA at day 10 (n = 9/group combined from 3 independent experiments). (C) ECs were FACS purified at day 7 and Bmp4 expression was measured by qPCR (n = 6/group combined from 2 independent experiments). (D-E) exECs were generated as previously described26,32 and stimulated for 24 hours with ZnSO4 at the indicated concentrations and Bmp4 expression measured by qPCR at 24 hours (50 μM: n = 8/group combined from 3 independent experiments; 100 μM: n = 11/group across 5 independent experiments) (D), and BMP4 protein was quantified by ELISA at 48 hours (n = 3 independent experiments) (E). (F) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given 550 cGy TBI. Mice were administered with the BMP type I receptor inhibitor dorsomorphin dihydrochloride (12.5 mg/kg) intraperitoneally at day −1 before TBI and twice daily after TBI, and all mice were maintained on ZnSO4 in drinking water for the duration of the study. Total thymus cellularity was quantified at day 10 after TBI (n = 5/group from 1 independent experiment). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Zn stimulates the production BMP4 by ECs. (A) 6- to 8-week-old female C57BL/6 mice were fed a normal or ZD diet for 21 days, at which point mice were given 550 cGy TBI. One cohort was given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) from day 0. Levels of BMP4 were quantified by ELISA at day 10 after TBI (n = 5-10/group from 1 independent experiment). (B-C) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given 550 cGy TBI. Mice were maintained on Zn-supplemented drinking water for the duration of the study. (B) BMP4 levels measured by ELISA at day 10 (n = 9/group combined from 3 independent experiments). (C) ECs were FACS purified at day 7 and Bmp4 expression was measured by qPCR (n = 6/group combined from 2 independent experiments). (D-E) exECs were generated as previously described26,32 and stimulated for 24 hours with ZnSO4 at the indicated concentrations and Bmp4 expression measured by qPCR at 24 hours (50 μM: n = 8/group combined from 3 independent experiments; 100 μM: n = 11/group across 5 independent experiments) (D), and BMP4 protein was quantified by ELISA at 48 hours (n = 3 independent experiments) (E). (F) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given 550 cGy TBI. Mice were administered with the BMP type I receptor inhibitor dorsomorphin dihydrochloride (12.5 mg/kg) intraperitoneally at day −1 before TBI and twice daily after TBI, and all mice were maintained on ZnSO4 in drinking water for the duration of the study. Total thymus cellularity was quantified at day 10 after TBI (n = 5/group from 1 independent experiment). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
To mechanistically interrogate the direct effect of Zn on thymic ECs, we constitutively activated the AKT pathway using the prosurvival adenoviral gene E4ORF1, which allows ECs from multiple tissues, including the thymus, to be propagated and manipulated ex vivo while maintaining their phenotype and vascular tube formation for functional manipulation and in vitro modeling of regenerative pathways.26,32,45 We found a dose-dependent increase in Bmp4 expression within ECs exposed ZnSO4 before a toxicity cliff, with a concentration of 100 µM of ZnSO4 leading to the most robust response with no evidence of toxicity (Figure 3D; supplemental Figure 4C). This finding was confirmed at the protein level after 48 hours of exposure to Zn (Figure 3E). Consistent with our hypothesis that Zn is involved in the in vivo pathway of BMP4 production, treatment with the pan-BMP-receptor inhibitor dorsomorphin dihydrochloride abrogated the effect of ZS on thymic regeneration (Figure 3F).
Extracellular translocation of Zn after acute damage stimulates production of BMP4 by ECs
Given the dual effects of Zn on T-cell development and the production of BMP4 by ECs, we wanted to clarify the endogenous role of Zn during thymic repair. To do this, we first measured changes in Zn levels in otherwise untreated wild-type mice after TBI by ICP-MS, an analytical technique that can be used to measure elements at trace levels in biological fluids.46 Although the total amount of Zn in whole tissue lysates (both intracellular and extracellular compartments) decreased after damage, following the same trend as thymic cellularity (Figure 4A),26 when we assessed extracellular Zn (using supernatants) as a function of total Zn, we found a significant translocation of Zn from intracellular to extracellular space after damage (Figure 4B).
Zn accumulates in thymocytes and is released after damage. (A-B) Six- to 8-week-old female C57BL/6 mice were given 550 cGy TBI, and levels of Zn were measured by ICP-MS. (A) Total thymic amounts of Zn from both intracellular and extracellular fractions of thymus (n = 6/timepoint). (B) Extracellular Zn was measured only in thymic supernatants, and the ratio of extracellular to total thymic Zn was calculated (n = 6/timepoint). (C) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point 1 cohort was given 550 cGy of TBI. Thymocytes were isolated either before or 48 hours after TBI and cocultured with exECs. Bmp4 expression was measured by qPCR at 24 hours (n = 3-4/group). (D) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for either 21 days before TBI or from the day of TBI and maintained on ZnSO4 in drinking water for the duration of the study. Thymus cellularity was measured at day 28 after TBI (n = 4-5/group). (E) Six- to 8-week-old female C57BL/6 mice were fed a normal or ZD diet for 21 days, after which thymocytes were isolated by CD90+ magnetic separation. Intracellular Zn levels were measured by staining with Fluozin-3 and assessed by flow cytometry (n = 5/group across 2 independent experiments). (F-G) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, after which thymocytes were isolated by CD90+ magnetic separation and Zn was measured by staining with Fluozin-3 (F) or ICP-MS (G). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Zn accumulates in thymocytes and is released after damage. (A-B) Six- to 8-week-old female C57BL/6 mice were given 550 cGy TBI, and levels of Zn were measured by ICP-MS. (A) Total thymic amounts of Zn from both intracellular and extracellular fractions of thymus (n = 6/timepoint). (B) Extracellular Zn was measured only in thymic supernatants, and the ratio of extracellular to total thymic Zn was calculated (n = 6/timepoint). (C) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point 1 cohort was given 550 cGy of TBI. Thymocytes were isolated either before or 48 hours after TBI and cocultured with exECs. Bmp4 expression was measured by qPCR at 24 hours (n = 3-4/group). (D) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for either 21 days before TBI or from the day of TBI and maintained on ZnSO4 in drinking water for the duration of the study. Thymus cellularity was measured at day 28 after TBI (n = 4-5/group). (E) Six- to 8-week-old female C57BL/6 mice were fed a normal or ZD diet for 21 days, after which thymocytes were isolated by CD90+ magnetic separation. Intracellular Zn levels were measured by staining with Fluozin-3 and assessed by flow cytometry (n = 5/group across 2 independent experiments). (F-G) Six- to 8-week-old female C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, after which thymocytes were isolated by CD90+ magnetic separation and Zn was measured by staining with Fluozin-3 (F) or ICP-MS (G). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
To functionally assess this finding, we cocultured exECs with supernatants isolated from ZS-treated mice at day 0 and 48 hours after TBI. We found increased expression of Bmp4 in ECs cocultured with supernatant from thymuses harvested 2 days after TBI (Figure 4C). Given that we observed a more robust effect if ZS was begun several weeks before TBI (Figure 4D), we hypothesized that thymocytes, which require Zn for their maturation, accumulate Zn during ZS, which allows for increased bioavailability of extracellular Zn after damage and the triggering of regenerative responses in ECs. Consistent with this hypothesis, thymocytes isolated from mice given a ZD diet exhibited significantly lower levels of Zn (Figure 4E), and mice given ZS in their drinking water showed significantly increased levels of intracellular Zn (Figure 4F-G).
Zn signals though GPR39 on ECs to stimulate production of BMP4
There are 2 main modalities by which Zn can mediate its effect on cells: influx (and efflux) using the ZIP (and ZnT) ion channels47,48 and via the cell surface Zn-sending G-protein coupled receptor, GPR39.49,50 To identify the putative mode action for Zn, we selectively increased intracellular Zn concentrations of exECs by treatment with the Zn ionophore sodium pyrithione. We did not observe any increase of Bmp4 expression after treatment with pyrithione (Figure 5A), suggesting that binding to a surface receptor is more likely than through Zn internalization. GPR39 could not be detected on thymocyte populations; however, we found significant expression on nonhematopoietic stromal cells such as TECs, fibroblasts, and ECs (Figure 5B; supplemental Figure 5A-C). Notably, we found an increase in expression of GPR39 on ECs (Figure 5C; supplemental Figure 5B), suggesting their potential to respond to extracellular Zn after damage is increased and consistent with reports demonstrating that EC function can be regulated by GPR39 signaling.49,50 GPR39 acts by translating extracellular Zn signals into release of intracellular second messengers such as ERK and calcium release.43,51 Consistent with this, when we blocked ERK with the inhibitor FR180204 prior to Zn stimulation in vitro, BMP4 production was abrogated (Figure 5D) and we found that ERK phosphorylation was significantly increased in situ at day 4 after TBI alone, with considerable colocalization of phosphorylated ERK and CD31, a marker of ECs (Figure 5E-F; supplemental Figure 5D-E). Importantly, demonstrating the functional importance of GPR39 for EC-mediated regeneration, Zn-mediated production of Bmp4 was abrogated in exECs after silencing of Gpr39 expression (Figure 5G; supplemental Figure 5F-G). Stimulation of exEC with the selective GPR39 agonist TC-G 100852 induced expression of Bmp4 greater than Zn alone, although there was no synergistic increase by combining Zn and TC-G1008 (Figure 5H), likely because they both target the same receptor.
GPR39 expressed by thymic ECs is the central mediator of Zn-centered regeneration. (A) exECs were stimulated for 24 hours with ZnSO4 (100 μM) with or without the Zn ionophor pyrythione. Bmp4 expression was measured by qPCR (n = 6 combined from 2 independent experiments). (B) GPR39 expression across subsets in the thymus by flow cytometry at baseline. Displayed are concatenated plots from 1 experiment. (C) Expression of GPR39 on cTECs, mTECs, ECs, and fibroblasts at days 0, 4, and 7 after TBI. Displayed are concatenated plots from 1 experiment. (D) exECs were stimulated for 24 hours with ZnSO4 (100 μM) with or without the ERK inhibitor FR180204. BMP4 was measured by ELISA. (E) ERK phosphorylation in situ assessed at steady state and at day 4 in thymus after TBI (550 cGy) in 6-week-old female C57BL/6 mice. pERK is shown in green, DAPI for nuclear staining in blue. (F) Average quantification of pERK intensity/region of interest (ROI) in thymuses at day 0 and day 4 after SL-TBI. (G) GPR39 expression was silenced in exECs by siRNA and stimulated for 24 hours with ZnSO4 (100 μM), after which Bmp4 expression was measured by qPCR (n = 3/group). (H) Bmp4 expression in exEC cultured for 24 hours in presence of ZnSO4 (100 μM) and/or the GPR39 agonist TC-G1008 (25 μM) (n = 5-15 combined from 5 independent experiments). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
GPR39 expressed by thymic ECs is the central mediator of Zn-centered regeneration. (A) exECs were stimulated for 24 hours with ZnSO4 (100 μM) with or without the Zn ionophor pyrythione. Bmp4 expression was measured by qPCR (n = 6 combined from 2 independent experiments). (B) GPR39 expression across subsets in the thymus by flow cytometry at baseline. Displayed are concatenated plots from 1 experiment. (C) Expression of GPR39 on cTECs, mTECs, ECs, and fibroblasts at days 0, 4, and 7 after TBI. Displayed are concatenated plots from 1 experiment. (D) exECs were stimulated for 24 hours with ZnSO4 (100 μM) with or without the ERK inhibitor FR180204. BMP4 was measured by ELISA. (E) ERK phosphorylation in situ assessed at steady state and at day 4 in thymus after TBI (550 cGy) in 6-week-old female C57BL/6 mice. pERK is shown in green, DAPI for nuclear staining in blue. (F) Average quantification of pERK intensity/region of interest (ROI) in thymuses at day 0 and day 4 after SL-TBI. (G) GPR39 expression was silenced in exECs by siRNA and stimulated for 24 hours with ZnSO4 (100 μM), after which Bmp4 expression was measured by qPCR (n = 3/group). (H) Bmp4 expression in exEC cultured for 24 hours in presence of ZnSO4 (100 μM) and/or the GPR39 agonist TC-G1008 (25 μM) (n = 5-15 combined from 5 independent experiments). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Activation of GPR39 signaling promotes T-cell reconstitution after HCT
Thymic regeneration is a particular challenge after the myeloablative conditioning required for successful HCT.53 We found that dietary ZS promoted thymic reconstitution in a minor-antigen mismatched model of murine T-depleted (TCD) allogeneic (allo)-HCT (where any effects mediated by GVHD can be excluded) (Figure 6A). Increased cellularity was observed in all developing thymocyte and TEC subsets (supplemental Figure 6A). To track the export of T cells from the thymus, we performed TCD allo-HCT in which donors expressed GFP under the control of RAG2, which allows for the detection of cells recently exported from the thymus (referred to as recent thymic emigrants [RTEs]).27,54 In both peripheral blood and spleen, mice that received ZS showed higher levels of both CD4+ and CD8+ RTEs and naïve T cells after HCT (Figure 6B; supplemental Figure 6B-C). However, the complicated mechanism by which dietary ZS promotes thymic regeneration involves prolonged treatment before HCT for thymocytes to accumulate Zn to be released after injury, thereby allowing signaling through GPR39 in regeneration-initiating ECs. To test if directly stimulating GPR39 could abrogate this lead time, we treated mice with the GPR39 agonist TC-G1008. Using this approach, we could show that mice treated with TC-G1008 showed significantly improved thymic function in models of both SL-TBI (Figure 6C) in mice given a TCD allo-HCT across multiple minor histocompatibility antigens (Figure 6D). Notably, in the HCT model we observed a significant increase in the export of CD4+ and CD8+RTEs, which was also reflected by an increased number of naïve T cells in mice treated with TC-G1008 (Figure 6E-F). Peripheral T cells from mice treated with either ZS or TC-G1008 were functionally active when stimulated in vitro with anti-CD3 and CD28 antibodies, exhibiting equivalent proliferation and production of interferon (IFN) γ, interleukin (IL) 2, and tumor necrosis factor (TNF) α (Figure 6G-H; supplemental Figure 6D-E). Additionally, the proportion of CD4+ and CD8+ subpopulations in lymph nodes did not differ between control and TC-G1008–treated mice (supplemental Figure 6F).
Experimental targeting of the GPR39 receptor improves thymic repair and T-cell reconstitution after allo-HCT. (A) Six- to 8-week-old male C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given a lethal dose of TBI (2 × 550 cGy) along with T-cell depleted BM from female C57BL/6 mice. Mice were maintained on ZnSO4 in drinking water for the duration of the study. Total thymic cellularity at days 7, 21, 28, and 42 after HCT (n = 5-10/group/timepoint combined from 2 independent experiments). (B) Six- to 8-week-old male C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given a lethal dose of TBI (2 × 550 cGy) along with T-cell depleted BM from female RAG2-GFP mice. Splenic T cells were analyzed for GFP expression on day 53 (n = 4-5/group). (C) Six- to 8-week-old female C57BL/6 mice were given 550 cGy of TBI and either vehicle or TC-G 1008 (20 mg/kg per mouse per day) by guided feeding daily from day 0 until day 10, when thymus cellularity was assessed (n = 8-9 combined from 2 independent experiments). (D-H) Six- to 8-week-old female BALB.B mice were given a lethal dose of TBI (900 cGy) along with 10 × 106 T-cell depleted BM from female C57BL/6 mice and either vehicle or TC-G 1008 (20 mg/kg per mouse per day) by guided feeding daily from day 0 until day 8 and then biweekly. Thymuses were harvested and analyzed at day 14 (n = 10/group from 2 separate experiments) and at day 40 (n = 6/group from 2 separate experiments)(D) Total thymic cellularity at day 14 and 40 after HCT. (E) Spleen harvested at day 40 after HCT, and numbers of GFP+ T cells were calculated (n = 6/group, from 2 separate experiments). (F) Number of T-cell subsets. (G-H) CD3+ T cells were isolated from spleen at day 40 after HCT and stimulated for 72 hours with anti-CD3 and anti-CD28. (G) Proliferation of all T cells (n = 6/group from 2 separate experiments). (H) Intracellular cytokine staining for IFN-γ, IL-2, and TNF-α on CD4+ and CD8+ (n = 6/group from 2 separate experiments). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Experimental targeting of the GPR39 receptor improves thymic repair and T-cell reconstitution after allo-HCT. (A) Six- to 8-week-old male C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given a lethal dose of TBI (2 × 550 cGy) along with T-cell depleted BM from female C57BL/6 mice. Mice were maintained on ZnSO4 in drinking water for the duration of the study. Total thymic cellularity at days 7, 21, 28, and 42 after HCT (n = 5-10/group/timepoint combined from 2 independent experiments). (B) Six- to 8-week-old male C57BL/6 mice were given supplemental Zn in drinking water (300 mg/kg per day of ZnSO4) for 21 days, at which point mice were given a lethal dose of TBI (2 × 550 cGy) along with T-cell depleted BM from female RAG2-GFP mice. Splenic T cells were analyzed for GFP expression on day 53 (n = 4-5/group). (C) Six- to 8-week-old female C57BL/6 mice were given 550 cGy of TBI and either vehicle or TC-G 1008 (20 mg/kg per mouse per day) by guided feeding daily from day 0 until day 10, when thymus cellularity was assessed (n = 8-9 combined from 2 independent experiments). (D-H) Six- to 8-week-old female BALB.B mice were given a lethal dose of TBI (900 cGy) along with 10 × 106 T-cell depleted BM from female C57BL/6 mice and either vehicle or TC-G 1008 (20 mg/kg per mouse per day) by guided feeding daily from day 0 until day 8 and then biweekly. Thymuses were harvested and analyzed at day 14 (n = 10/group from 2 separate experiments) and at day 40 (n = 6/group from 2 separate experiments)(D) Total thymic cellularity at day 14 and 40 after HCT. (E) Spleen harvested at day 40 after HCT, and numbers of GFP+ T cells were calculated (n = 6/group, from 2 separate experiments). (F) Number of T-cell subsets. (G-H) CD3+ T cells were isolated from spleen at day 40 after HCT and stimulated for 72 hours with anti-CD3 and anti-CD28. (G) Proliferation of all T cells (n = 6/group from 2 separate experiments). (H) Intracellular cytokine staining for IFN-γ, IL-2, and TNF-α on CD4+ and CD8+ (n = 6/group from 2 separate experiments). Graphs represent mean ± SEM; each dot represents a biologically independent observation. *P < .05; **P < .01; ***P < .001.
Taken together, these data suggest that improved thymic regeneration caused by Zn signaling can help immune reconstitution after HCT by increasing the production of thymic-derived naïve T cells. Additionally, this pathway can be pharmacologically targeted by stimulating GPR39 signaling.
Discussion
Alterations in Zn uptake, retention, sequestration, or secretion can quickly lead to ZD and affect Zn-dependent functions in virtually all tissues, particularly in the immune system, including thymic involution.16,23,24 We show that even short-term Zn deprivation in young animals has a profound impact on thymic function before effects on peripheral immune cells can be detected. ZD reduced the replicative ability of thymocytes, especially in the transition to DP thymocytes. Although the specific mechanism by which Zn acts on thymocyte development is unclear, there is evidence that many Zn-finger transcriptional factors that heavily depend on general Zn availability are crucial for T-cell development.55-57 In our data, intracellular Zn levels in thymocytes responded rapidly to changes in systemic Zn availability, with lower levels of Zn in thymocytes under ZD and higher levels after ZS. This is consistent with the notion that T cells actively internalize Zn during activation and replication through the expression of Zn importers such as ZIP6.21 Notably, the changes observed in thymic function preceded the other classic signs of ZD, such as weight loss and skin and fur changes, confirming the sensitivity of thymopoiesis to Zn level although the contribution of systemic effects cannot be completely ruled out.58
Thymic regeneration is a complex and poorly understood process in which the cytokines produced from damage-resistant cells, such as IL-22 from innate lymphoid cells, IL-23 from dendritic cells, and BMP4 from ECs, stimulate TECs to proliferate and mediate broader thymic repair.26,31 BMP4 is a member of bone morphogenic proteins, a family of peptides involved in embryogenesis and homeostasis of many tissues,59 including thymic organogenesis and maintenance of Foxn1 expression in TECs.41,60,61 We found that modulating levels of Zn in the thymus using either ZD or ZS had a concomitant effect on BMP4 expression. Furthermore, stimulation of thymic ECs with ZnSO4 directly induced the production of BMP4 in a GPR39-dependent manner, and the administration of a BMP receptor inhibitor abrogated the effect of ZS on thymic repair. Notably, a similar role for Zn release into the extracellular space after acute damage has been demonstrated to be involved in tissue repair in tissues such as skin and gut.17,62-64
The G-protein coupled receptor GPR39 was recently discovered as a “Zn sensing receptor”43 with putative roles in tissue repair in the gut and skin.65,66 However, while Zn is involved in epithelial cell function in other organs67-69 and TECs do express GPR39, our data suggest that its role in TEC regeneration after acute injury is likely indirect through BMP4 although we cannot exclude the possibility that GPR39 also mediates effects directly on other stromal cells. Therefore, we can conclude that Zn is needed not only for thymocyte maturation but also for thymic repair after acute damage by stimulating the production of BMP4 by ECs. However, because we have also recently identified an alternate pathway that leads to upregulation of BMP4 (centered on attenuation of the detection of dying thymocytes by TAM receptors after damage), we hypothesize that Zn sensing of GPR39 is not the only mechanism responsible for triggering tissue repair.70
Thymic regeneration is important after myeloablative conditioning required for successful HCT, after which there is prolonged suppression of T-cell immunity. The importance of finding strategies to stimulate thymic-dependent immune reconstitution is highlighted by the correlation between RTEs and clinical outcomes after HCT.1,9 Given its effects on T-cell development and the induction of regenerative factors, it was perhaps not surprising that ZD mice exhibited worse repair after TBI; however, the fact that ZD led to even worse recovery in mice that had thymic GVHD highlighted its importance for restoration of thymic function after acute damage and highlighted the potential for clinically targeting this pathway. Importantly, we could show improved thymic function after allogeneic HCT and show that this enhanced repair was translated into the circulation with increased numbers of RTEs. This fits with the results from a pilot clinical trial that our group had performed in patients receiving autologous HCT.71 However, our findings suggest that the therapeutic benefit of dietary ZS demands an extended pretreatment for thymocytes to accumulate Zn. Therefore, our findings suggest that directly targeting the GPR39 receptor itself with a pharmacological agonist is an attractive alternative to induce an equivalent reparative response when given at the time of myeloablative conditioning. Indeed, treatment of ECs with a small molecule agonist for GPR39 led to significantly enhanced production of BMP4, even greater than stimulation with Zn, and exogenous treatment of mice led to significantly enhanced thymic repair.
In conclusion, these findings highlight the importance of Zn in steady-state T-cell development and reveal a role for Zn in endogenous tissue repair. The studies outlined here not only define important pathways underlying tissue regeneration but could also result in innovative clinical approaches to enhance T-cell reconstitution in recipients of HCT.
Acknowledgments
The authors gratefully acknowledge Eugenio Mocchegiani, Marco Malavolta, and Robertina Giacconi of INRCA (Istituto Nazionale di Ricovero e Cura per Anziani), Ancona, for their feedback about this project, the Flow Cytometry and Comparative Medicine Shared Resources at the Fred Hutchinson Cancer Research Center, and the TraceLab in the School of Oceanography at the University of Washington, for ICP-MS. The authors also gratefully acknowledge Juan-Carlos Zuniga-Plucker (University of Toronto) for the gift of OP9-DL1 cells.
This study was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (grant R01-HL145276) (J.A.D.), Project 2 of P01-AG052359 (J.A.D.), and the National Cancer Institute Cancer Center Support (grant P30-CA015704). Support was also received from a Scholar Award from the American Society of Hematology (J.A.D.), The Rotary Foundation through a Global Grant (L.I.), and the Immunotherapy Integrated Research Center at the Fred Hutchinson Cancer Research Center (L.I.).
Authorship
Contribution: M.P., L.I., and J.A.D. conceived the idea for this manuscript; J.A.D. and L.I. designed, analyzed, and interpreted experiments and drafted the manuscript; K.C., P.d., S.K., C.E., D.G., C.S., and K.H. performed experiments; A.G. and T.U. performed ICP-MS; F.M. performed multidimensional analyses of flow cytometry data; G.R.H. and K.S.E. helped design, perform, and interpret the GVHD experiments; S.G. helped revise the manuscript; and all authors contributed to the article and approved the submitted version.
Conflict-of-interest disclosure: G.R.H. has consulted for Generon Corporation, NapaJen Pharma, iTEOS Therapeutics, and Neoleukin Therapeutics and receives research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, and iTeos Pharmaceuticals. L.I. and J.A.D. have a patent application pending around these findings to promote thymus regeneration. The remaining authors declare no competing financial interests.
Correspondence: Lorenzo Iovino, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: liovino@fredhutch.org; and Jarrod A. Dudakov, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: jdudakov@fredhutch.org.
No high throughput datasets were generated for this manuscript. Requests for data sharing may be submitted to Lorenzo Iovino (liovino@fredhutch.org) and Jarrod A. Dudakov (jdudakov@fredhutch.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal