In this issue of Blood, Sakemura et al1 demonstrate that cancer-associated fibroblasts (CAFs) inhibit CAR T-cell antimyeloma activity and devise a strategy to overcome CAF-induced CAR T-cell inhibition by using CAR T cells to target both multiple myeloma and CAFs.
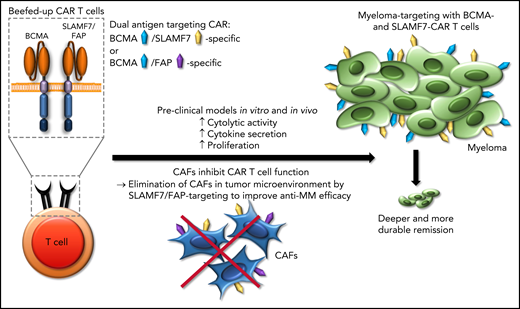
Chimeric antigen receptor (CAR) T-cell therapy for myeloma is under intense investigation. CAR T cells that target B-cell maturation antigen (BCMA) as a single antigen have been approved for relapsed or refractory multiple myeloma,2 but the depth of remission and the durability of responses are modest, given the effort required and expense of this complex therapy. One strategy that has been proposed to augment the antimyeloma efficacy of CAR T-cell therapy is dual-antigen targeting of BCMA and other myeloma antigens such as signaling lymphocyte activation molecule, family member 7 (SLAMF7), GPRC5D, and CD19, to eradicate a greater number of myeloma cells and to prevent myeloma escape through antigen downmodulation and loss.3,4 Sakemura et al have taken a new angle on dual-antigen targeting by seeking to both target the tumor cells and eliminate the CAFs that create the myeloma niche in the bone marrow. T cells directed against BCMA in combination with either SLAMF7 or fibroblast-associated protein (FAP) confer substantially increased antimyeloma efficacy in preclinical models in vitro and in vivo (see figure). Indeed, these beefed-up CAR Ts confer increased cytolytic activity, cytokine secretion, and proliferation against multiple myeloma cells in the presence of CAFs compared with conventional CAR T cells that are directed against BCMA as a single antigen. The beefed-up CAR Ts induce deeper and more durable remissions in xenograft models with multiple myeloma cell lines and CAFs that have been isolated from the bone marrow of patients with myeloma.
Beefed-up CAR T cells. Applying dual-antigen targeting (either BCMA/SLAMF7 or BCMA/FAP) inhibits CAFs in the tumor microenvironment and, thus, increases the anti–multiple myeloma (anti-MM) efficacy of CAR T cells that target BCMA.
Beefed-up CAR T cells. Applying dual-antigen targeting (either BCMA/SLAMF7 or BCMA/FAP) inhibits CAFs in the tumor microenvironment and, thus, increases the anti–multiple myeloma (anti-MM) efficacy of CAR T cells that target BCMA.
Several prior studies have provided insights into the potential role of CAFs in inhibiting the efficacy of CAR T-cell therapy: first, by creating a physical barrier that impairs T-cell migration and infiltration of tumor lesions, and second, as an immunological barrier related to the production of a plethora of secreted cytokines and membrane-bound ligands that act as negative regulators of T-cell function.5,6 Sakemura et al investigated the obvious candidates TGF-β and PD-L1 as examples of secreted and membrane-bound ligands and found only a minor impact of those on CAR T-cell function, suggesting that additional cytokines and ligands are the mediators of the inhibitory CAF effect exerted on CAR T-cell function. Additional study of the attributes of CAFs obtained from patients with myeloma at different disease stages are warranted to further dissect the negative mechanisms and to provide additional and more refined strategies to neutralize their inhibitory effect.
The approach presented by Sakemura et al is at the same time radical, simple, and effective, directed at eliminating CAFs in the myeloma bone marrow niche through the CAR T-cell product. The expression of FAP on CAFs has been documented in previous studies in solid tumors and early-stage clinical trials of FAP-specific CAR T cells have been opened.7 The expression of SLAMF7 on CAFs comes as a surprise as the expression of SLAMF7 is highly restricted to the hematopoietic system, with expression of SLAMF7 on subsets of normal T, B, and NK cells, as well as malignant plasma cells in multiple myeloma.8 Of particular interest, the data presented by Sakemura et al suggest that SLAMF7 is expressed on myeloma-associated CAFs in the bone marrow, but not on bone marrow stroma in healthy donors. They suggest 2 candidates for clinical development: BCMA/FAP- and BCMA/SLAMF7-specific CAR T cells. The appeal of targeting of BCMA and SLAMF7 is the increased therapeutic pressure against myeloma cells through elimination of myeloma-associated CAFs. Additional work is needed to determine the efficacy of this approach for eliminating myeloma in the bone marrow and extramedullary lesions and in the central nervous system, a privileged and difficult site to tackle.
The anticipated safety profile of targeting BCMA and SLAMF7 should be benign, as shown by the clinical experience with the SLAMF7-specific antibody elotuzumab and the emerging data from clinical trials with SLAMF7-specific CAR T cells.9 However, in light of recent data with FAP-specific CAR T cells for treating cardiac fibrosis, targeting of FAP seems an attractive and feasible strategy. The data reported by Sakemura et al encourage the continued investigation of dual-antigen targeting in multiple myeloma. CAR T cells are amenable to genetic modification, with 2 (and potentially more) CAR constructs which, from a practical and cost perspective, seems favorable compared with the administration of 2 antibodies, antibody drug conjugates, or T-cell–engaging antibodies.10 The clinical trial landscape with CAR-T cells in multiple myeloma is extremely busy; however, if the improvement in response rate and durability of response observed in the preclinical setting holds true in the clinical setting, these beefed-up CAR Ts certainly have a bright future.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal