Key Points
Exposure to systemic corticosteroid is associated with hospitalization for VOEs in patients with SCD.
Systemic corticosteroids should be avoided in patients with SCD.
Abstract
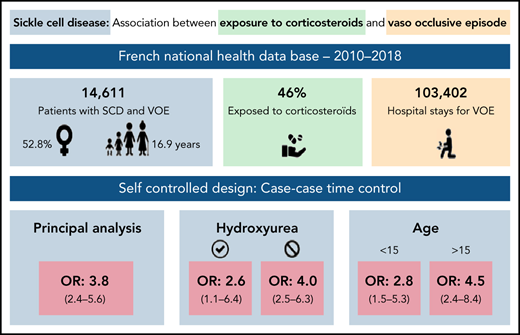
Vaso-occlusive episodes (VOEs) are a major concern in patients with sickle cell disease (SCD). Exposure to systemic corticosteroids has been suspected to increase the occurrence of VOEs in case reports or series. No comparative study has been conducted to investigate this risk, which is still debated. Several clinical trials demonstrated the effectiveness of corticosteroids for the treatment of VOEs, but with increased rates of readmission. The aim of the study was to assess the risk of hospitalization for VOE associated with exposure to systemic corticosteroids in patients with SCD. We used a case-case-time-control design in a nationwide population-based cohort built in the French national health insurance database between 2010 and 2018. The population included all patients with SCD with at least 1 hospitalization for VOE. Corticosteroids were identified using out-of-hospital dispensing data. The outcome was the first hospitalization for VOE. The case-case-time-control design induces self-adjustment for time-invariant confounders, including genotype. Analyses were adjusted for time-dependent confounders (infections, red blood transfusions) and stratified by exposure to hydroxyurea. Overall, 5151 patients were included in the main analysis. Corticosteroid exposure was significantly associated with the occurrence of hospitalizations for VOEs: adjusted odds ratio, 3.8; 95% confidence interval [CI], 2.4-5.6). In patients exposed to hydroxyurea, the adjusted odds ratio was 2.6 (95% CI, 1.1-6.4); it was 4.0 (95% CI, 2.5-6.3) in unexposed patients. These results were consistent in children and adults. In conclusion, systemic corticosteroids were associated to an increased risk of hospitalization for VOEs and should be limited in patients with SCD.
Introduction
Sickle cell disease (SCD) is the most frequent monogenic disease in the world. It affects approximately 300 000 newborns per year.1,2 Incidence and prevalence depend on geographical areas, with a predominance in sub-Saharan Africa, the Mediterranean basin, and India.3
Patients with SCD present recurrent episodes of pain called acute vaso-occlusive crisis or vaso-occlusive episode (VOE), which is the leading cause of care seeking in this population and an important cause of morbidity and mortality.4-8 Some risk factors of VOEs are well known, such as infection9 and hypoxemia (eg, pulmonary infection, altitude).10,11
Exposure to systemic corticosteroids (oral and injectable) has been suspected to increase the occurrence of VOEs. This finding is based on several case reports and series.9,10,12-16 Vaso-occlusive episodes have been also described in patients following intra-articular injection of corticosteroids,17 but not with exposure to inhaled corticosteroids.18 Noteworthy, the use of corticosteroids as a treatment for VOEs is also debated: 2 randomized trials showed a beneficial effect on VOEs (particularly on acute chest syndrome) with reduced length of hospital stay and of the number of red blood transfusion,19,20 whereas 2 retrospective studies showed a rebound effect with an increased rate of rehospitalization in patients with acute chest syndrome treated with corticosteroids.21,22
The risk of VOE associated with systemic corticosteroids has not been measured in comparative studies and is still debated. Therefore, given the lack of clear recommendations regarding the prescription of corticosteroids in patients with SCD, corticosteroids are still widely prescribed in this population.
Consequently, this study aimed to assess the risk of hospitalization for VOE associated with out-of-hospital exposure to systemic corticosteroids in patients with SCD.
Methods
Data source
The study was conducted using data from the French national health insurance system database, the Système National des Données de Santé (SNDS; National Health Data System). This database prospectively records individualized, anonymous, and linkable data for the entire French population (66.9 million individuals). Data collected include demographics (age, sex, city of residence, vital status, and date of death), out-of-hospital data (including all dispensing of reimbursed drugs), and hospital data. The hospital database contains data of all hospital stays of both private and public hospitals and includes admission and discharge dates, discharge diagnoses (1 primary, 1 related, and several associated diagnoses) coded with the International Classification of Diseases, 10th version (ICD-10), and procedures (ie, red blood transfusions).23-25
Study population
The study population was the national cohort of all patients with SCD (adults and children) hospitalized at least once for VOE between January 2010 and December 2018 in France. They were identified using the ICD-10 code D57.0 (“sickle-cell anemia with crisis”) as the primary hospital discharge diagnosis code. Although it is recommended to use this code for SS-SCD with crisis, there is no other available code for VOE in the ICD-10 used in the SNDS. In practice, all VOEs, including VOEs occurring in patients with double-heterozygous SCD, are encoded using this code in France.26 Therefore, all genotypes were included in our study population. This diagnosis code has a positive predictive value of 98.6% (95% confidence interval [CI], 92.3-100.0) in the French hospital database.26
Study design
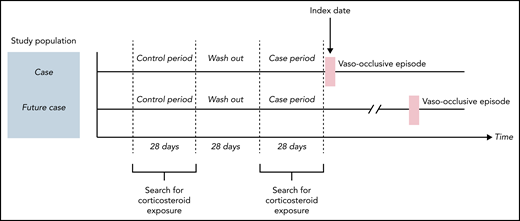
For the principal analysis, we performed a case-case-time-control (CCTC) analysis. This design was first described by Wang in 2011.27 The CCTC design is an extension of the case-crossover (CCO) design.28-30 The CCO design is a self-controlled design in which, among patients who experienced the event of interest (cases), the frequency of exposure during a period preceding immediately the event (case period) is compared with the frequency of exposure during 1 (or several) earlier period (control period). In case of greater exposure in the case period, this favors a potentially inducing role of the exposure in the event occurrence. The use of self-controlled designs induces self-adjustment on time-invariant characteristics. However, the CCO design can be affected by a bias resulting from temporal variation (annual, seasonal) in the exposure, named exposure trend, which is important with corticosteroids. To best control for this exposure trend, it is recommended to use an adjustment cohort with a probability of exposure similar to cases. Thus, we used a matched cohort of futures cases (patients with SCD with VOE the year after). In the CCTC design, 2 concomitant CCO analyses are conducted: 1 among the “cases” and 1 among “future cases” (Figure 1). The ratio (odds ratio [OR] in cases/OR in future cases) allows us to obtain the OR associated with the effect of the exposure on the event by taking into account the exposure trend.
Study design of principal analysis: case-case-time-control. Two concomitant case-crossover (CCO) analyses were conducted: 1 among the “cases” and 1 among “future cases.” Cases were patients with VOE (stripped bar, index date) during the study period. Corticosteroids exposure is expected to vary by seasonal periods, resulting in a variable probability of exposure over time. To best control for this exposure trend, using an adjustment cohort with a probability of exposure similar to cases is recommended. This adjustment cohort is made of “future cases.” They were patients with VOE the year after the index date (VOE of the case), matched with cases on age, sex, and residence place. In each CCO analysis, the frequency of exposure during the case period (28 days before VOE) was compared with the frequency of exposure during the control period (also with a duration of 28 days, separated from the case period by a 28-day washout period). The ratio (OR in cases/OR in future cases) gives the OR associated with the effect of the exposure on the event adjusted for the exposure trend.
Study design of principal analysis: case-case-time-control. Two concomitant case-crossover (CCO) analyses were conducted: 1 among the “cases” and 1 among “future cases.” Cases were patients with VOE (stripped bar, index date) during the study period. Corticosteroids exposure is expected to vary by seasonal periods, resulting in a variable probability of exposure over time. To best control for this exposure trend, using an adjustment cohort with a probability of exposure similar to cases is recommended. This adjustment cohort is made of “future cases.” They were patients with VOE the year after the index date (VOE of the case), matched with cases on age, sex, and residence place. In each CCO analysis, the frequency of exposure during the case period (28 days before VOE) was compared with the frequency of exposure during the control period (also with a duration of 28 days, separated from the case period by a 28-day washout period). The ratio (OR in cases/OR in future cases) gives the OR associated with the effect of the exposure on the event adjusted for the exposure trend.
Outcome
The outcome was the first hospitalization for VOE identified during the study period. The index date was the day of admission to hospital.
Exposure of interest
Exposure to corticosteroids during case and control periods was searched within out-of-hospital dispensing data using the Anatomical Therapeutic Chemical classification code H02AB (“corticosteroids for systemic use-glucocorticoids”) including oral systemic corticosteroids (prednisone, prednisolone, hydrocortisone, betamethasone, methylprednisolone, and dexamethasone), intravenous corticosteroids (hydrocortisone, methylprednisolone, and betamethasone), and corticosteroids used for intra-articular administration (betamethasone, prednisolone, triamcinolone, and cortivazol). A patient was classified as “exposed” when the drug was dispensed during the given period of interest. Topical and inhaled corticosteroids were not included. Given the nature of the database, in-hospital exposures to corticosteroids were not recorded.
Confounding factors
Potential confounding factors are described in the directed acyclic graph in supplemental Figure 1 on the Blood Web site. Sex, age, SCD genotype, residence area, and socioeconomic status are potential time-independent confounding factors that are self-adjusted with the CCTC design.
Exposure to hydroxyurea was searched within out-of-hospital dispensing data using the corresponding Anatomical Therapeutic Chemical code (supplemental Table 1). A patient was classified as exposed to hydroxyurea if he or she was dispensed it at least once during the 3 months before the index date.
Exposure to antibiotics was used as a proxy of infection and searched within out-of-hospital dispensing data (supplemental Table 1). We excluded phenoxymethylpenicillin, recommended as a prophylactic treatment for patients with SCD in France.
Exposure to red blood transfusion was searched among daily hospital stays (supplemental Table 1).
As in exposure to corticosteroids, exposure to antibiotics and red blood transfusion were searched in case and control periods.
Analytic methods
Principal analysis
The case period was defined as the 28 days before the index date. We have chosen a duration of 28 days because, in France, outpatient dispensing of drugs covers a maximum duration of treatment of 28 days. The index date was not included in the case period. The control period, of the same duration, started 84 days before the VOE of the case. We used a washout period of 28 days between these 2 periods (Figure 1).
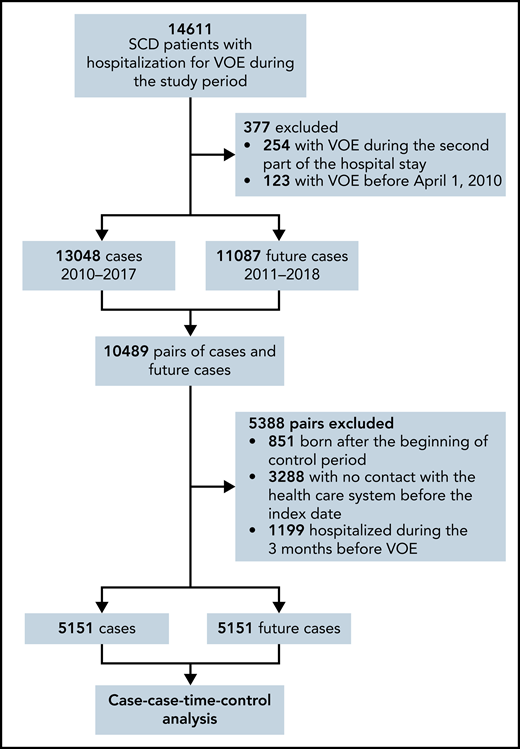
Cases and future cases were matched by age (±3 years), sex, and residence area (mainland France vs overseas territories). Futures cases were patients presenting with VOE in the year following the VOE of the corresponding case. Each patient could be “case” and “future case” only once. Because we aimed to assess out-of-hospital exposure to corticosteroids, cases hospitalized more than 24 hours during the 84 days before the index date were excluded as were their corresponding future cases. We also excluded the patients with no contact with the health care system before the index date to avoid bias in case of out-of-France unmeasured exposure to corticosteroids.
Sensitivity analysis
We performed several sensitivity analyses. First, by varying the study design: 2 CCO designs with control periods 84 days and 1 year before VOE, respectively (supplemental Figure 2B and C, respectively), and 1 case-time control design with general population control (matched by age, sex, and residence area) to adjust for exposure trend in the general population (supplemental Figure 2D). Second, we conducted a sensitivity analysis using the same model as the principal analysis (CCTC) but using shorter case and control periods of 14 days (supplemental Figure 2E).
Statistical analysis
For all analyses, we performed conditional logistic regression models. Time-independent confounders were self-adjusted by self-controlled designs. Analyses were adjusted for exposure to antibiotics, red blood transfusions, and stratified according to exposure to hydroxyurea. We conducted subgroup analyses by age at the first VOE observed in the study period (<15 vs ≥15 years old) and by sex. The 15-year cutoff was chosen because, in France, from the age of 15 years, patients are managed by adult specialists and no longer by pediatricians, which may imply a different management. Adjusted odds ratios (aOR) and their 95% CIs were computed. All analyses were performed using SAS Guide (SAS Institute, Cary, NC, USA).
Ethics
This study was authorized by the French institute of health data (Institut des Données de Santé, number 2990547) and by the French personal data protection authority (Commission nationale de l’informatique et des libertés, number 921170).
Results
Population and outcome
Between 2010 and 2018, 14 166 patients with SCD were hospitalized for VOEs (Table 1), with 103 204 hospitalizations for VOE in total (of which 13.0% were in intensive care units), corresponding to 0.78 VOEs per patient-year. The median duration of hospital stay for VOEs was 3 days (interquartile range [IQR], 1-4 days).
Characteristics of patients hospitalized for VOE during the study period and of cases included in the principal analysis
| Characteristics . | Patients hospitalized for VOE during the study period . | Cases included in the principal analysis . |
|---|---|---|
| Number of patients | 14 611 | 5,151 |
| Women, no. (%) | 7665 (52.5) | 2778 (53.9) |
| Median age at first VOE (IQR), y | 16.7 (5.6-28.8) | 16.9 (6.3-31.2) |
| Died during the study period (%), no. | 442 (3.0) | 152 (2.9) |
| Residence area | ||
| Mainland France, no. (%) | 11 985 (82.0) | 4015 (78.0) |
| Overseas territories, no. (%) | 2626 (18.0) | 1136 (22.0) |
| Characteristics . | Patients hospitalized for VOE during the study period . | Cases included in the principal analysis . |
|---|---|---|
| Number of patients | 14 611 | 5,151 |
| Women, no. (%) | 7665 (52.5) | 2778 (53.9) |
| Median age at first VOE (IQR), y | 16.7 (5.6-28.8) | 16.9 (6.3-31.2) |
| Died during the study period (%), no. | 442 (3.0) | 152 (2.9) |
| Residence area | ||
| Mainland France, no. (%) | 11 985 (82.0) | 4015 (78.0) |
| Overseas territories, no. (%) | 2626 (18.0) | 1136 (22.0) |
We selected from the overall population 5151 cases and futures cases for the principal analysis (Figure 2; Table 1).
Flowchart illustrating the selection of patients for the principal analysis.
Exposure to corticosteroids
Forty-five percent of the 5151 patients were exposed at least once to systemic corticosteroids between 2010 and 2018, including 317 (6.2%) exposed during the case period in the principal analysis (supplemental Table 2). Oral corticosteroids represented 95.4% of systemic corticosteroids. The median time between corticosteroids dispensing and the index date was 5 days (IQR, 3-9 days). Variation over time of corticosteroid dispensing among cases and general population-matched controls (sensitivity analysis using the case-time control design) are presented in supplemental Figure 3.
Exposure to confounding
During the study period, 92.3% of the patients included in the study population had at least 1 exposure to antibiotics and 60.7% received at least 1 red blood transfusion. In the principal analysis, 748 cases (14.5%) received antibiotics and 226 (4.4%) received red blood transfusions during the case period. Forty-three percent of the patients with hospitalization for VOE during the study period were exposed at least once to hydroxyurea, including 616 cases (12.0%) included in the principal analysis during the 3 months before the index date (supplemental Table 2).
Comparative analyses
Associations between out-of-hospital exposure to systemic corticosteroids and hospitalization for VOE are presented in Table 2. In the principal analysis, the aOR was 3.8 (95% CI, 2.4-5.6). In patients exposed to hydroxyurea, the aOR was 2.6 (95% CI, 1.1-6.4); it was 4.0 (95% CI, 2.5-6.3) in unexposed patients. The aOR was 2.8 (95% CI, 1.5-5.3) in children and 4.5 (95% CI, 2.4-8.4) in adults. It was 6.5 (95% CI, 3.5-12.3) in women and 2.1 (95% CI, 1.1-4.0) in men. Results were consistent in all sensitivity analyses (Table 2).
Association between out-of-hospital systemic corticosteroids and hospitalization for VOE: result of principal, subgroup, and sensitivity analyses
| . | . | . | Multivariate analysis by exposure to HU . | |
|---|---|---|---|---|
| . | Univariate analysis . | Multivariate analysis . | Adjusted OR* (95% CI) . | |
| Crude OR (95% CI) . | Adjusted OR* (95% CI) . | With HU . | Without HU . | |
| Principal analysis | ||||
| All patients (N = 5151) | 4.8 (3.1-7.4) | 3.8 (2.4-5.6) | 2.6 (1.1-6.4) | 4.0 (2.5-6.3) |
| Subgroup analysis | ||||
| Age ≥15 y (n = 2743) | 5.6 (3.0-10.5) | 4.5 (2.4-8.4) | NA | NA |
| Age <15 y (n = 2408) | 3.5 (1.5-6.6) | 2.8 (1.5-5.3) | NA | NA |
| Men (n = 2373) | 2.7 (1.5-5.1) | 2.1 (1.1-4.0) | NA | NA |
| Women (n = 2778) | 7.9 (4.3-14.6) | 6.5 (3.5-12.3) | NA | NA |
| Sensitivity analyses | ||||
| CTC† (n = 4725) | 4.9 (3.4-7.1) | 3.7 (2.5-5.4) | 3.4 (1.3-8.7) | 3.7 (2.5-5.6) |
| CCO‡ (n = 10 648) | 3.8 (3.1-4.5) | 3.0 (2.5-3.6) | 3.1 (1.7-5.8) | 3.0 (2.4-3.7) |
| CCO§ (n = 8852) | 5.2 (4.1-6.5) | 4.4 (3.5-5.5) | NA | NA |
| CCTC‖ (n = 5746) | 6.5 (4.0-10.8) | 4.7 (2.8-7.9) | NA | NA |
| . | . | . | Multivariate analysis by exposure to HU . | |
|---|---|---|---|---|
| . | Univariate analysis . | Multivariate analysis . | Adjusted OR* (95% CI) . | |
| Crude OR (95% CI) . | Adjusted OR* (95% CI) . | With HU . | Without HU . | |
| Principal analysis | ||||
| All patients (N = 5151) | 4.8 (3.1-7.4) | 3.8 (2.4-5.6) | 2.6 (1.1-6.4) | 4.0 (2.5-6.3) |
| Subgroup analysis | ||||
| Age ≥15 y (n = 2743) | 5.6 (3.0-10.5) | 4.5 (2.4-8.4) | NA | NA |
| Age <15 y (n = 2408) | 3.5 (1.5-6.6) | 2.8 (1.5-5.3) | NA | NA |
| Men (n = 2373) | 2.7 (1.5-5.1) | 2.1 (1.1-4.0) | NA | NA |
| Women (n = 2778) | 7.9 (4.3-14.6) | 6.5 (3.5-12.3) | NA | NA |
| Sensitivity analyses | ||||
| CTC† (n = 4725) | 4.9 (3.4-7.1) | 3.7 (2.5-5.4) | 3.4 (1.3-8.7) | 3.7 (2.5-5.6) |
| CCO‡ (n = 10 648) | 3.8 (3.1-4.5) | 3.0 (2.5-3.6) | 3.1 (1.7-5.8) | 3.0 (2.4-3.7) |
| CCO§ (n = 8852) | 5.2 (4.1-6.5) | 4.4 (3.5-5.5) | NA | NA |
| CCTC‖ (n = 5746) | 6.5 (4.0-10.8) | 4.7 (2.8-7.9) | NA | NA |
CTC, case-time-control; HU, hydroxyurea; NA, not applicable.
Adjusted by out-of-hospital antibiotics and red-blood-cell transfusions.
CTC with matched general population controls.
CCO with the control period 84 days before the index date.
CCO with the control period 1 year before the index date.
CCTC with case and control periods of 14 days.
Discussion
This nationwide population-based study showed an increased risk of hospitalization for VOE after out-of-hospital exposure to systemic corticosteroids. The strength of the association was lower in men, in children, and in patients treated with hydroxyurea.
Patients’ characteristics were in line with the epidemiology of SCD. More than 100 000 stays for VOE for 14 611 patients were identified, 13% of which required a stay in the intensive care unit, confirming the frequency and severity of VOEs. The median number of VOEs per patient-year identified was in line with clinical prospective studies.31,32 Noteworthy, 45% of the population was exposed at least once to systemic corticosteroids during the study period, stressing the need of clear recommendations about their use.
Subgroup analyses according to sex found a risk of VOE that appeared to be higher in women than in men, despite an overlap in CIs. This difference is probably not related to gynecological or obstetrical issues because the difference is also observed in patients younger than 15 years of age (data not shown). In our cohort, women had more dispensing of corticosteroids than men (62.4% of corticosteroid dispensing was for women), and more frequent hospital stays for VOE (51.4% of stays were for women). In accordance, some studies have found a greater propensity to develop VOEs in women than in men.33 In addition, the pharmacological effects of corticosteroids differ according to sex,34 highlighting the need of studies to explore the pathophysiological hypothesis underlying these findings.
The median time between corticosteroid dispensing and hospitalization for VOE was 5 days. The pathophysiology of the increased risk of VOE with exposure to corticosteroids is uncertain. Because leukocytosis is associated with VOE,6 the frequent increase in neutrophil count by corticosteroids could be a leading factor, and the beneficial effect of hydroxyurea could be due to limitation of leukocytosis. Other authors mention a rebound effect when corticosteroids are stopped because of the end of the membrane stabilizing the effect of corticosteroids and the release of inflammatory mediators.35 Another hypothesis is that bone necrotic emboli by osteonecrosis induced by corticosteroids would be responsible for VOEs, according to a concept of retrograde embolization.35,36
One of the strengths of the study is the nationwide inclusion of patients between 2010 and 2018, with a high number of patients exposed to systemic corticosteroids. Self-controlled designs are appropriate methods to measure this risk because of self-adjustment on time-independent confounding factors. Fundamental hypotheses of these pharmacoepidemiological designs were respected.37 Case and control periods were close to limit the risk of time-dependent confounding bias. The CCTC design is adjusted for the exposure trend, which is a potential major bias in CCO designs.27,38-40 We observed slight differences of variations over time of out-of-hospital systemic corticosteroid dispensing between patients with SCD and general population controls (supplemental Figure 3). As a result, the CCTC seems more appropriate than the case-time control design in this setting. All sensitivity analyses using these other designs and with variation of case, washout, and control periods resulted in a high consistency of the results, suggesting the robustness of the association (Table 2).
Our study has several limitations. Regarding the data source, we could not distinguish the different genotype types of major SCD (SS, SC, Sβ+, or Sβ0).37 Because 79% of patients with SCD in France are SS-SCD,41 extrapolation of the results seems possible in these patients, but we cannot affirm that the risk is also present for the less represented genotypes, in particular SC and Sβ+ patients, considered to have a less severe disease. Similarly, we could not identify acute chest syndrome because of the absence of ICD-10-specific codes available in the SNDS during the study period. Therefore, we cannot draw a conclusion on the association between the exposure to systemic corticosteroids and the occurrence of acute chest syndrome; this association is particularly debated in this subgroup of patients.19-22,42 We studied patients residing in France, which limits the extrapolation of results to all patients with SCD worldwide, especially in low-income countries. Because of the nature of the database, we could not identify VOEs in out-of-hospital patients. However, severe VOEs requiring hospitalization are those responsible for major morbidity and are therefore the subgroup of most interest.4
Conversely, because cases hospitalized more than 24 hours during the 84 days before the index date were excluded, patients very frequently hospitalized could have been excluded. Because our study was limited to the period between 2010 and 2018, the first VOE identified was not necessarily the first VOE of included patients. Yet, VOE occurrences are not independent, and we can hypothesize that a hospitalization for VOE before our study period altered the likelihood of subsequent exposure to corticosteroids (because of awareness of this possible risk by specialized physicians). However, the high proportion of patients exposed to systemic corticosteroids during the study period indicates that this risk was not well-known. Channeling bias in clinical practice seems unlikely. All analyses were adjusted for antibiotic dispensing to minimize this risk in case of coprescription of corticosteroids and antibiotics for an infection leading to VOE. Indeed, the prescription of corticosteroids alone (without antibiotics) for an infection in this population with hyposplenism is improbable. We had no information about indication of corticosteroids and could not assess the risk by indications. The database provides information on the number of tablets dispensed, but not on the dose and the duration of the treatment prescribed to the patient. Thus, we were not able to assess a dose-effect relationship or any possible rebound effect. The exposure to corticosteroids was measured by dispensing data, which could differ from the effective intake by patients. However, systemic corticosteroids are prescribed for acute conditions. Consequently, a significant unmeasured time from dispensing to intake is unlikely. Last, we could not assess the association by subgroups of children treated vs untreated by hydroxyurea because of the low number of children exposed to hydroxyurea, as well as in the small subgroup of patients treated with chronic transfusion.
In conclusion, this study sustains that systemic corticosteroids were associated with increased risk of hospitalization for VOE and should be limited in patients with SCD.
Acknowledgments
The authors thank the Société Nationale Française de Médecine Interne, Recherche et Enseignement en Médecine Interne, and Toulouse University Hospital.
Authorship
Contribution: O.W., P.C., J.M., P.B., M.L.-M., M.L., and G.M. designed the study; O.W. conducted the statistical analyses; O.W., M.L., and G.M. interpreted the data; O.W., M.L., and G.M. wrote the paper. All authors reviewed the manuscript and gave final approval. They agree to be accountable for all aspects of the work. G.M. is the guarantor.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ondine Walter, Centre d’Investigation clinique CIC 1436, Centre Hospitalier Universitaire de Toulouse, France, Place du Docteur Baylac-TSA 40031-31059 Toulouse cedex 9, France; e-mail: walter.o@chu-toulouse.fr.
Data may be obtained from a third party and are not publicly available. The data of the SNDS are anonymous. They can be accessed by submitting a request to the Health-Data-Hub at https://www.health-data-hub.fr/depot. The data management and statistical analysis code is available on reasonable request from the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal