In this issue of Blood, Ambrosio et al1 identify 3 proteins and complexes that contribute to α-granule biogenesis, yielding insights into how the myriad of cargo is packaged into this abundant class of platelet granules.
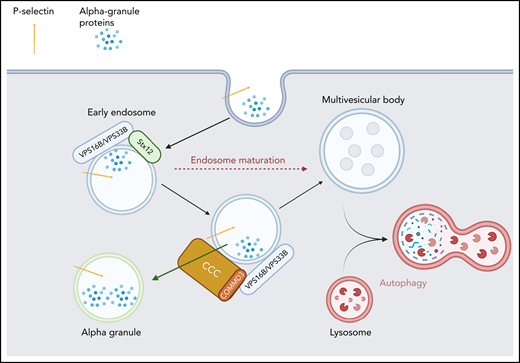
Studies of gray platelet syndrome (MIM 139090) and arthrogryposis–renal dysfunction–cholestasis syndrome (MIM 208085) identified NBEAL2 and VPS33B/VPS16B, respectively, as gene products important for α-granule production in megakaryocytes (MKs). Ambrosio et al expanded those findings, identifying syntaxin 12 (STX12; aka syntaxin 13) as interacting with VPS33B/VPS16B. They further showed how STX12 and a sorting complex called the COMMD (copper metabolism MURR1 domain)–CCDC22 (coiled-coil domain-containing 22)–CCDC93 (CCC) complex compete for VPS33B/VPS16B binding, suggesting a progressive hand-off mechanism for cargo sorting. Deletion of STX12, CCDCC22, or COMMD3 reduced α-granule numbers and cargo levels in immortalized megakaryocyte progenitor cell lines (imMKCLs). These deletions also increased the number of multivesicular bodies (MVBs), which are thought to be an intermediate in α-granule biogenesis. Selective deletion of these proteins, as well as sorting nexin 17 (SNX17), delineated the trafficking route for P-selectin, showing that the α-granule membrane protein is retrieved from the plasma membrane and sorted, in the endosomes, to nascent granules. These observations increase our list of players in α-granule biogenesis and solidify the importance of endosomes in packaging de novo synthesized and endocytosed cargo into the same granules.
VSP33B and VSP16B (aka C14orf133, VIPAR, SPE-39) have several potential binding partners.2 Because VSP33B is a member of the Sec1/Munc18 family of Qa-soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor chaperones, Ambrosio et al first asked which of the 13 human syntaxin (STX) gene products bound recombinant VPS33B/VSP16B complexes. Pull-down and coimmunoprecipitation experiments confirmed robust interactions between STX12 and VPS33B/VSP16B. Interestingly, a phosphomimetic mutation in the Habc domain of STX12 enhanced binding, suggesting regulation of the “closed” configuration of STX12 to control this interaction. Modulation of STX12 levels by small interfering RNA or clustered regularly interspaced short palindromic repeats decreased the number of α-granules and the levels of 3 α-granule cargoes (von Willebrand factor [vWF], platelet factor 4 [PF4], and P-selectin). STX12 was localized to endosomes and partially colocalized with the key sorting protein, SNX17, which also recognizes P-selectin.3
Previous proteomic studies identified CCDC22 as a VSP33B/VSP16B interactor.2 Ambrosio et al expanded those observations, showing that STX12 and CCDC22 competed for binding to a specific site on VSP33B. This interaction was shown to be functionally important for α-granule biogenesis using rescue experiments in VPS33B−/− cells. They further showed that deletion of CDCC22 in imMKCLs reduced α-granule numbers, as well as PF4 and P-selectin levels. Together with a COMMD family member and CCDC93, CCDC22 forms a larger complex (called the CCC complex), which is important for endosome cargo sorting. Ambrosio et al found that 3 of the 13 COMMD genes (COMMD3, COMMD5, COMMD7) were upregulated as imMKCLs differentiate. Deletion of COMMD3 reduced α-granule numbers and PF4 and P-selectin levels. Treating the cells with the vacuolar H+ ATPase inhibitor, bafilomycin A1, reversed this reduction, suggesting that the loss of COMMD3 leads to a missorting of cargo to the lysosome for degradation. Interestingly, COMMD3 and STX12 show partial colocalization on a tubular organelle that could be the tubular recycling endosome, which is known to be a hub of anterior and retrograde protein trafficking.4 Such partial colocalization might be expected if sequential interactions in membrane microdomains of this compartment are required for cargo sorting.
This study represents a landmark advance in our understanding of α-granule biogenesis and opens the field to further dissection of the process. These studies reveal much about how cargo gets to an α-granule. STX12, SNX17, and CCC complexes are part of the endosomal sorting machinery. They interact with the cytoplasmic tails of endocytosed membrane proteins and direct them to various compartments (ie, the plasma membrane, Golgi apparatus; see figure). As shown by Ambrosio et al, loss of these elements leads to cargo degradation in an acidic compartment, presumably the lysosome. Does this imply that many α-granule proteins are secreted first and then recovered from the extracellular space via endocytosis? For PF4 and P-selectin, such a pathway is consistent with the data presented, as well as that from other groups.5,6 Because PF4 is almost exclusively expressed by MKs, a significant portion of it, and perhaps other factors, must not be immediately stored in α-granules post-Golgi but instead undergo exocytosis before reuptake and packaging. Several proteins (eg, vWF and TSP1) produced by the MK are found in early endosome (RAB5+) structures alongside cargo that is known to be endocytosed (eg, FGN).7 This pathway might give MKs more flexibility to change the cargo composition of nascent platelets and make granule content packaging more responsive to the microenvironmental changes in the bone marrow.
α-Granule protein trafficking through endosomal compartments. Some α-granule proteins are endocytosed into early endosomes where sorting occurs. STX12 is required for membrane fusion to deliver that cargo. The VPS16B/VPS33B complex initially binds STX12 and then may be handed off to the CCC complex. CCC facilitates retrieval of these proteins from the endosomal system and directs them to α-granules.
α-Granule protein trafficking through endosomal compartments. Some α-granule proteins are endocytosed into early endosomes where sorting occurs. STX12 is required for membrane fusion to deliver that cargo. The VPS16B/VPS33B complex initially binds STX12 and then may be handed off to the CCC complex. CCC facilitates retrieval of these proteins from the endosomal system and directs them to α-granules.
An additional question focuses on the sorting of soluble nonmembrane protein cargo. The system described by Ambrosio et al is used for membrane proteins. Are there membrane proteins whose cytoplasmic tails interact with the sorting machinery and whose luminal domains select soluble cargo for packaging? Are these proteins cargo specific, or is there a more general process driven by charge-charge interactions, as suggested for the granule scaffold protein serglycin?5 Understanding this packaging scheme will help us to devise methods to specifically load MKs and, thus, platelets with therapeutic molecules that would be releasable upon platelet activation.
Finally, what other proteins are involved in α-granule biogenesis, and how do they work together? NBEAL2 interactors have been identified8; how do they work with the VPS33B/VSP16B interactors? The identification of STX12 suggests that the machinery selects SNAREs needed for the membrane fusion required for intercompartmental transit. VPS16B has been shown to bind to the R-SNARE VAMP-7.9 Perhaps these sorting complexes select cargo, as well as direct the formation of membrane-fusing trans-SNARE complexes, to assure correct delivery of cargo. The work of Ambrosio et al answers several of the existing questions and identifies many new routes for future investigation into how MKs package the diverse array of α-granule cargo.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal