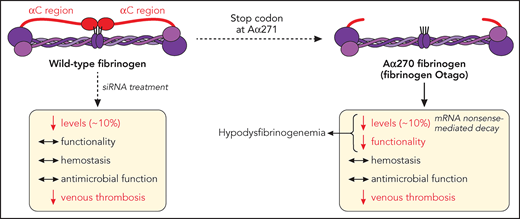
In this issue of Blood, Hur et al1 expand our understanding of hypodysfibrinogenemia, where a naturally occurring variant (fibrinogen Otago) exhibits normal hemostatic and antimicrobial functions, while protecting against thrombosis, in mice (see figure).
Fibrinogen α-chain truncation at Arg271 leads to a shortened αC region, a naturally occurring mutation (fibrinogen Otago) leading to hypodysfibrinogenemia (reduced levels and function). This mutation does not dramatically affect hemostasis (bleeding) or fibrinogen-mediated survival to microbial infection, but reduces the development of venous thrombosis, in mice. Similarly, siRNA-induced reduction of circulating fibrinogen levels to ∼10% retain fibrinogen functionality, hemostatic and antimicrobial functions, while still preventing venous thrombosis. mRNA, messenger RNA. Professional illustration by Patrick Lane, ScEYEnce Studios.
Fibrinogen α-chain truncation at Arg271 leads to a shortened αC region, a naturally occurring mutation (fibrinogen Otago) leading to hypodysfibrinogenemia (reduced levels and function). This mutation does not dramatically affect hemostasis (bleeding) or fibrinogen-mediated survival to microbial infection, but reduces the development of venous thrombosis, in mice. Similarly, siRNA-induced reduction of circulating fibrinogen levels to ∼10% retain fibrinogen functionality, hemostatic and antimicrobial functions, while still preventing venous thrombosis. mRNA, messenger RNA. Professional illustration by Patrick Lane, ScEYEnce Studios.
Congenital hypodysfibrinogenemia is characterized by reduced plasma levels of fibrinogen alongside altered function, mostly caused by mutations in the fibrinogen Aα chain.2 However, the mechanisms underpinning the reduced circulating fibrinogen levels, and the relevance of inherent effects of the mutation vs those of the reduced fibrinogen levels on the hemostatic/thrombotic balance, are still unknown.
On one hand, afibrinogenemia (no circulating fibrinogen) is associated with severe spontaneous bleeding from all tissues, ranging from umbilical cord to the central nervous system, with potentially fatal intracranial hemorrhage, although hypofibrinogenemia (low levels of circulating fibrinogen) is usually asymptomatic in its mild and moderate forms, with virtually no unprovoked bleeding and normal pregnancy.2 On the other hand, dysfibrinogenemia (abnormal form of circulating fibrinogen), although mostly asymptomatic, presents with risks of developing major bleeding and/or cardiovascular events.3 The question remains as to whether levels of circulating fibrinogen are a driving force to several pathological conditions. In contrast to afibrinogenemia and hypofibrinogenemia exhibiting bleeding tendencies, elevated circulating fibrinogen levels are associated with increased risk of cardiovascular disease, such as coronary heart disease and stroke.4 Paradoxically, afibrinogenemic patients2 and fibrinogen-deficient mice5 present with an increased risk of thromboembolism, attributed to embolization of unstable platelet clots not stabilized by fibrin.
In their article, Hur and colleagues demonstrate that reduction of fibrinogen level to ∼10% of normal circulating levels is sufficient to maintain hemostasis and resistance to infection, while convincingly reducing thrombosis (see figure).
First, the authors describe a transgenic mouse strain (Fga270) replicating a naturally occurring mutation (Otago, fibrinogen Aα chain [Fga] truncation at residue 271), to unravel the mechanisms by which mutations to the fibrinogen Aα chain lead to reduced circulating plasma levels. The Fga270 phenotype is similar to that of human hypodysfibrinogenemia and provides the first concrete evidence that nonsense-mediated decay is responsible for decreased levels of protein expression, because treatment of Fga270 hepatocytes with cycloheximide increased Fga messenger RNA levels.
Next, the authors showed that small interfering RNA (siRNA) injection in wild-type mice (Fga10%) reduced circulating levels of normal fibrinogen to that of Fga270 mice (∼10%), therefore allowing to dissect the impact of low level, vs mutant form, of fibrinogen on the hemostatic/thrombotic balance. Here, low levels of fibrinogen, with(out) Fga mutations, was sufficient to sustain a normal bleeding phenotype, despite Fga270 mice forming clots that are less dense with thicker fibers, with both Fga270 and Fga10% plasma exhibiting impaired clotability. To explain this discrepancy, some compensatory mechanisms were proposed, where fibrinolysis was reduced in abnormal Fga270 plasma clots, because of the loss of the Aα271-610 region, which is required for the binding of fibrinolytic proteins, while Fga270 and Fga10% platelets exhibited preserved platelet-fibrinogen interaction leading to normal platelet aggregation but faster disaggregation. The normal bleeding phenotype in FgaWT, Fga270, and Fga10%, vs the lack of hemostasis in Fga-deficient mice, is in agreement with studies in hypofibrinogenemic patients showing that those with circulating fibrinogen levels >1 mg/mL (normal range, 2 to 4 mg/dL) remained asymptomatic, whereas decreasing fibrinogen levels correlated with increasing bleeding severity.6
Importantly, an inferior vena cava ligation model used in this article demonstrates that both Fga270 and Fga10% mice are protected against venous thrombosis, similarly to Fga-deficient mice, attributing these findings to the lack of fibrin Aα-chain cross-linking responsible for red blood cell retention in the clot and the (lack of) circulating fibrinogen levels, respectively. The treatment of choice for afibrinogenemic patients is prophylactic long-term fibrinogen supplementation with plasma-derived fibrinogen concentrate, with some of those patients found to develop thrombosis following infusion. This study potentially indicates that supplementation toward a lower range of circulating fibrinogen may reduce the risk of thrombosis in those patients.
In addition to hemostasis and thrombosis, fibrinogen also plays a role in host defense against pathogen infection, where afibrinogenemic patients exhibit abnormal skin test reactions to several microbial antigens,7 and fibrinogen-deficient mice display reduced survival to bacterial infection.8 Here, Hur and colleagues use a Staphylococcus aureus peritoneal infection model to demonstrate that regardless of the fibrinogen Aα chain truncation, a reduction to 10% circulating fibrinogen levels is sufficient to sustain antimicrobial functions, because fibrinogen-mediated bacteria clearance and host survival remained unaffected.
Although this study provides insights into the mechanisms leading to reduced levels of fibrinogen in hypodysfibrinogenemia related to Aα-chain mutations, and some evidence that partial reduction of fibrinogen levels may provide antithrombotic effects without affecting risks of bleeding, the situation is likely more complex. Hence, further investigations should be carried into (1) the role of reduced fibrinogen levels on (i) rebleeding events, (ii) arterial thrombus formation, (iii) thromboembolization in both the venous and arterial systems; (2) the highest fibrinogen levels threshold required to still observe normal hemostasis and reduced thrombosis risks.
Although elevated fibrinogen levels are associated with neurological disorders, such as Alzheimer disease and vascular dementia,9 a link between fibrinogen levels and cancer is also emerging, with fibrinogen-deficient mice showing reduced primary colon tumor growth.10 Therefore, the model of reduction in circulating fibrinogen levels presented in this study may also offer opportunities to further define the role of fibrinogen in other diseases.
Lower therapeutic doses of plasma fibrinogen concentrate, and fibrinogen-lowering drugs, have therefore therapeutic potentials for the treatment of congenital fibrinogen disorders and the prevention of cardiovascular diseases and others, respectively.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal