Key Points
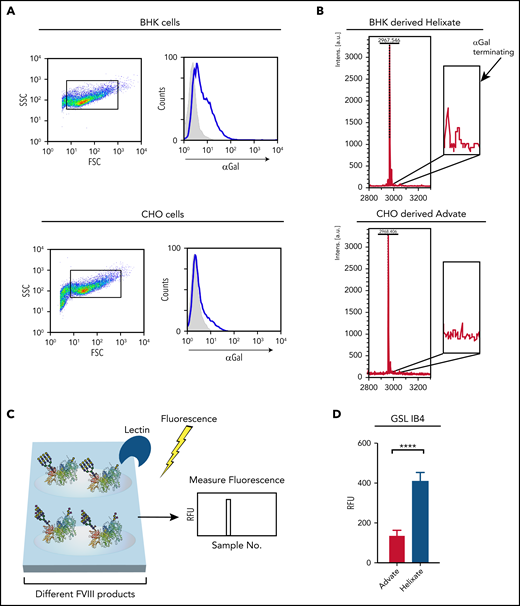
BHK-derived FVIII possesses higher levels of nonhuman αGal compared with CHO-derived FVIII.
BHK-derived FVIII exhibits increased reactivity with serum from αGal-KO mice and increased immunogenicity in αGal-KO recipients.
Abstract
Recombinant factor VIII (FVIII) products represent a life-saving intervention for patients with hemophilia A. However, patients can develop antibodies against FVIII that prevent its function and directly increase morbidity and mortality. The development of anti-FVIII antibodies varies depending on the type of recombinant product used, with previous studies suggesting that second-generation baby hamster kidney (BHK)-derived FVIII products display greater immunogenicity than do third-generation Chinese hamster ovary (CHO)-derived FVIII products. However, the underlying mechanisms responsible for these differences remain incompletely understood. Our results demonstrate that BHK cells express higher levels of the nonhuman carbohydrate α1-3 galactose (αGal) than do CHO cells, suggesting that αGal incorporation onto FVIII may result in anti-αGal antibody recognition that could positively influence the development of anti-FVIII antibodies. Consistent with this, BHK-derived FVIII exhibits increased levels of αGal, which corresponds to increased reactivity with anti-αGal antibodies. Infusion of BHK-derived, but not CHO-derived, FVIII into αGal–knockout mice, which spontaneously generate anti-αGal antibodies, results in significantly higher anti-FVIII antibody formation, suggesting that the increased levels of αGal on BHK-derived FVIII can influence immunogenicity. These results suggest that posttranslational modifications of recombinant FVIII products with nonhuman carbohydrates may influence the development of anti-FVIII antibodies.
Introduction
Patients who suffer from hemophilia A, an X-linked bleeding disorder that is characterized by a deficiency or absence of blood coagulation factor VIII (FVIII), often receive FVIII protein replacement therapy for the treatment or prevention of bleeding.1 Although this approach can decrease patient morbidity and mortality, patients who receive FVIII replacement can develop alloantibodies to FVIII that often eliminate its effectiveness.2,3 Although the advent of alternative treatment strategies for FVIII replacement in patients with inhibitors is promising,4 inhibitors continue to make it difficult to optimally manage bleeding patients and can directly increase patient morbidity, mortality, and overall cost of care.5-7
Previous studies suggest that a variety of genetic and environmental factors likely influence the development of anti-FVIII antibodies in patients.8-10 However, recent studies suggest that, in addition to patient characteristics, distinct recombinant FVIII products may intrinsically possess different levels of immunogenicity. More specifically, several studies suggest that second-generation FVIII products, which are derived recombinantly in baby hamster kidney (BHK) cells, can result in statistically significant increases in inhibitor development compared with third-generation recombinant FVIII products produced in Chinese hamster ovary (CHO) cells.11 However, the underlying mechanisms responsible for the increased immunogenicity of second-generation FVIII products remain incompletely understood. Some of the most unique alterations that a glycoprotein can experience following recombinant expression in distinct cell lines are posttranslational modifications.12,13 Among glycan modifications that can impact immunogenicity, the α1-3galactose (αGal) terminal modification, which does not occur in humans because of loss of activity of the glycosyltransferase responsible for its synthesis,14,15 is expressed in all lower mammals at varied levels. Because humans do not express this antigen, naturally occurring anti-αGal antibodies develop, presumably as a result of stimulation by microbial flora.16 Anti-αGal antibodies are a major barrier to xenotransplantation and are implicated in a variety of pathologies, including αGal syndrome, an immunoglobulin E anti-αGal antibody-mediated allergy to red meat that is precipitated by tick bites.17,18 Because these antibodies may also influence the immunogenicity of αGal-bearing proteins and BHK and CHO cells are derived from lower mammals, variable incorporation of the nonhuman αGal epitope may lead to increased immunogenicity observed among second-generation products compared with third-generation FVIII products.
Study design
BHK or CHO cells were examined for αGal expression by Griffonia simplicifolia lectin I isolectin B4 (IB4).19,20 FITC-IB4 lectin staining cytometric analysis using a FACSCalibur was done as previously described.21 Determination of N-glycan composition for BHK-derived (Helixate) or CHO-derived (ADVATE) FVIII was achieved by matrix assisted laser desorption ionization time of flight analysis (Bruker).22 Each FVIII product was printed on a nitrocellulose microarray slide, followed by interrogation with IB4, serum, or antibody eluate and quantitative analysis (Scan Array Express; PerkinElmer Lifer Sciences).23,24 Levels of anti-αGal antibodies were determined by flow crossmatch using αGal+ red blood cells (RBCs), as done previously for the evaluation of other alloantibodies.25 Anti-αGal antibodies were absorbed using αGal+ RBCs, followed by antibody elution using standard procedures.26 Wild-type (WT) or αGal-knockout (KO) recipients received 4 weekly injections of Helixate or ADVATE (2 μg,) followed by examination of anti-FVIII antibody formation by enzyme-linked immunosorbent assay (ELISA), as outlined previously27 (see supplemental Methods for additional details, available on the Blood Web site).
Results and discussion
To determine whether levels of αGal expression differ between BHK and CHO cells, we first examined αGal antigen expression by flow cytometric analysis with IB4, a lectin that is commonly used to detect the αGal antigen.19,20 Using this approach, BHK cells displayed a higher level of αGal antigen than did CHO cells (Figure 1A), suggesting that BHK-derived FVIII may likewise possess higher levels of αGal. Because previous studies have reported variable αGal levels on CHO cells,28-31 and IB4 may exhibit some binding toward other glycans (http://www.functionalglycomics.org), the very low level of IB4 binding observed toward CHO cells may not reflect actual αGal expression or production of FVIII with αGal. To directly examine this, BHK- and CHO-derived FVIII products were subjected to mass spectrometric analysis for the αGal antigen. Consistent with αGal expression on BHK cells and the relative lack of αGal on CHO cells, αGal was observed on glycans harvested from FVIII derived from BHK cells but not CHO cells (Figure 1B), similar to previous analyses of FVIII glycosylation patterns.32
BHK-derived FVIII possesses higher levels of the αGal antigen than does CHO-derived FVIII. (A) BHK or CHO cells were incubated with FITC-IB4 lectin, followed by examination of IB4 binding by flow cytometric analysis. Blue lines represent IB4 binding, and gray shaded areas indicate no stain control. (B) Mass spectrometry analysis for αGal bearing N-glycans from BHK-derived FVIII (Helixate) or CHO-derived FVIII (ADVATE). Highlighted peaks correspond to biantennary N-glycans decorated with 1 or 2 αGal residues. (C) Schematic diagram of microarray construction, interrogation, and analysis for examining possible lectin and antibody interactions with BHK-derived or CHO-derived FVIII. (D) Quantitative analysis of IB4 binding following incubation with a microarray populated with ADVATE and Helixate. ****P < .0001, Student t test. FSC, forward scatter; RFU, relative fluorescence unit; SSC, side scatter.
BHK-derived FVIII possesses higher levels of the αGal antigen than does CHO-derived FVIII. (A) BHK or CHO cells were incubated with FITC-IB4 lectin, followed by examination of IB4 binding by flow cytometric analysis. Blue lines represent IB4 binding, and gray shaded areas indicate no stain control. (B) Mass spectrometry analysis for αGal bearing N-glycans from BHK-derived FVIII (Helixate) or CHO-derived FVIII (ADVATE). Highlighted peaks correspond to biantennary N-glycans decorated with 1 or 2 αGal residues. (C) Schematic diagram of microarray construction, interrogation, and analysis for examining possible lectin and antibody interactions with BHK-derived or CHO-derived FVIII. (D) Quantitative analysis of IB4 binding following incubation with a microarray populated with ADVATE and Helixate. ****P < .0001, Student t test. FSC, forward scatter; RFU, relative fluorescence unit; SSC, side scatter.
To determine whether the increased levels of αGal observed by mass spectrometry reflect accessible αGal at the protein surface, we next probed FVIII directly for αGal. To accomplish this, we printed BHK-derived FVIII and CHO-derived FVIII in a microarray format (Figure 1C), followed by analysis with IB4. Using this approach, we found that BHK-derived FVIII possessed significantly more reactivity by IB4 than did CHO-derived FVIII (Figure 1D), strongly suggesting that the αGal antigen present on BHK-derived FVIII is accessible and, therefore, may influence the development of inhibitors following BHK-derived FVIII exposure.
Naturally occurring anti-αGal antibodies are believed to facilitate immune responses to αGal-containing glycoproteins.33-35 However, because WT mice express the αGal antigen they are tolerized to this antigen and, therefore, do not spontaneously generate anti-αGal antibodies. To overcome this limitation, we used αGal-KO mice, which are deficient in the α1-3 galactosyltransferase and, therefore, do not generate the αGal antigen.36 To determine whether αGal-KO mice spontaneously generate anti-αGal antibodies, we first examined anti-αGal antibody levels in αGal-KO mice vs WT mice. Unlike WT mice, αGal-KO mice readily and spontaneously produced anti-αGal antibodies (Figure 2A), demonstrating a lack of immunological tolerance to the αGal antigen. To determine whether these naturally occurring antibodies can recognize FVIII, we incubated serum from αGal-KO mice with each FVIII in our microarray format. Serum isolated from αGal-KO mice displayed enhanced reactivity toward BHK-derived FVIII compared with CHO-derived FVIII (Figure 2B), suggesting increased reactivity to the αGal antigen. To determine whether the increased reactivity is due to αGal-reactive antibodies, we examined serum specifically absorbed and then eluted from αGal+ RBCs (Figure 2C). There was an increased reactivity toward BHK-derived FVIII that was completely eliminated by serum absorption on αGal+ RBCs, whereas the eluate restored the specific increase in reactivity toward BHK-derived FVIII (Figure 2D). These results suggest that low-level IB4 binding to CHO cells and CHO-derived FVIII likely reflects αGal-independent recognition and that, consistent with mass spectrometric analysis, anti-αGal antibodies specifically react with BHK-derived FVIII.
BHK-derived FVIII exhibits increased reactivity with αGal-KO serum and increased immunogenicity in αGal-KO mice. (A) Anti-αGal antibodies were evaluated by flow crossmatch analysis using αGal+ RBCs. Results are reported as mean fluorescence intensity (MFI). (B) Incubation of serum from αGal-KO mice with microarray containing BHK-derived FVIII (Helixate) or CHO-derived FVIII (Advate), followed by analysis of bound antibody. (C) Schematic diagram outlining the method used to absorb and elute αGal-specific antibodies from the serum of αGal-KO mice. (D) Evaluation of serum preabsorption, postabsorption on αGal RBCs, and antibody eluate obtained following elution of serum specifically absorbed onto αGal+ RBCs. (E) Evaluation of anti-FVIII antibody levels by ELISA following injection of BHK-derived FVIII into WT or αGal-KO recipients. (F) Evaluation of anti-FVIII antibody levels by ELISA following injection of CHO-derived FVIII into WT or αGal-KO recipients. ***P < .001, ****P < .0001, Student t test (A-B,E-F); ***P < .001, ****P < .0001, 1-way analysis of variance with Tukey’s post test (D). ns, not significant; RFU, relative fluorescence unit.
BHK-derived FVIII exhibits increased reactivity with αGal-KO serum and increased immunogenicity in αGal-KO mice. (A) Anti-αGal antibodies were evaluated by flow crossmatch analysis using αGal+ RBCs. Results are reported as mean fluorescence intensity (MFI). (B) Incubation of serum from αGal-KO mice with microarray containing BHK-derived FVIII (Helixate) or CHO-derived FVIII (Advate), followed by analysis of bound antibody. (C) Schematic diagram outlining the method used to absorb and elute αGal-specific antibodies from the serum of αGal-KO mice. (D) Evaluation of serum preabsorption, postabsorption on αGal RBCs, and antibody eluate obtained following elution of serum specifically absorbed onto αGal+ RBCs. (E) Evaluation of anti-FVIII antibody levels by ELISA following injection of BHK-derived FVIII into WT or αGal-KO recipients. (F) Evaluation of anti-FVIII antibody levels by ELISA following injection of CHO-derived FVIII into WT or αGal-KO recipients. ***P < .001, ****P < .0001, Student t test (A-B,E-F); ***P < .001, ****P < .0001, 1-way analysis of variance with Tukey’s post test (D). ns, not significant; RFU, relative fluorescence unit.
To determine whether the αGal antigen on BHK-derived FVIII may influence FVIII immunogenicity, we first sought to remove αGal antigen by enzymatic cleavage. However, attempts to remove αGal failed, because the conditions required for efficient αGal removal also rendered the FVIII protein inactive, likely as a result of protein denaturation. As a result, we instead sought to simply compare the immunogenicity of BHK-derived FVIII following injection into αGal-KO or WT mice to directly examine whether αGal influences inhibitor development. Using this approach, αGal-KO or WT recipients received multiple injections of BHK-derived FVIII, followed by examination for anti-FVIII antibodies by ELISA. Anti-FVIII antibody levels were significantly increased following BHK-derived FVIII injection into αGal-KO mice compared with WT recipients (Figure 2E). To determine whether similar differences in the immunogenicity occur following injection of CHO-derived FVIII into αGal-KO vs WT recipients, we next examined the immune response following injection of CHO-derived FVIII. In contrast to the results observed following BHK-derived FVIII injection, no difference in the development of anti-FVIII antibodies was observed following CHO-derived FVIII exposure (Figure 2F).
These results suggest that the increased levels of αGal on BHK-derived FVIII result in increased anti-αGal antibody binding and anti-FVIII antibody formation. These results have implications for the development of optimal FVIII products and therapeutic glycoproteins in general. Although the protein sequence of a recombinant glycoprotein may be identical or nearly identical to a human-derived glycoprotein, use of nonhuman cell lines to generate large quantities of recombinant glycoproteins may inadvertently result in the incorporation of nonhuman glycans that can directly impact the immunogenicity of the therapeutic product. These results suggest that analysis of FVIII glycosylation should be a key component of manufacturing quality control, especially when considering that metabolic variations and genetic drift can impact the glycosylation of a given cell.13 Furthermore, hemophilia patients exposed to tick bites who are at risk for red meat allergies may also experience an increased probability of developing anaphylactic reactions following second-generation FVIII exposure.17,18 Although these results do not suggest that nonhuman glycan incorporation is entirely responsible for the development of anti-FVIII antibodies following recombinant FVIII exposure, they do suggest that such a modification may influence FVIII immunogenicity and possibly other adverse events following FVIII exposure. As a result, careful consideration and characterization of the distinct cell sources, which may differ in their levels of αGal expression,14,28-31 and, more importantly, the level of αGal on FVIII products themselves, may be beneficial when seeking to produce optimal FVIII products for patients with hemophilia A.
Acknowledgments
The Emory Comprehensive Glycomics Core assisted with mass spectrometry analyses.
This work was supported, in part, by Hemophilia of Georgia and the Hemophilia of Georgia Center for Bleeding and Clotting Disorders Research Funding; the National Hemophilia Foundation–Shire Clinical Fellowship and Hemostasis and Thrombosis Research Society/Novo Nordisk Mentored Research Award in Hemophilia (P.E.Z.); the Burroughs Wellcome Trust Career Award for Medical Scientists, the Rare Bleeding Disorders (P.E.Z.); National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development Child Health Research Career Development Award K12HD072245; Atlanta Pediatric Scholars Program (P.E.Z.); NIH, Early Independence grant and NIH, National Heart, Lung, and Blood Institute grants HL141981 (S.R.S.) and R01HL154034 (C.M.A.), and HL141335 (P.L., S.R.S., and S.M.).
Authorship
Contribution: C.M.A., P.E.Z., and S.R.S. designed the research study, which was carried out with the support of S.S., J.W., X.S., C.B.D., P.L. and S.M.; C.M.A., P.E.Z., and S.R.S. also wrote the manuscript, which was edited and commented on by the remaining authors.
Conflict-of-interest disclosure: S.L.M. is a consultant for CSL Behring (Helixate producer) and Shire/Takeda (ADVATE producer). A National Hemophilia Foundation (NHF) award (P.E.Z.) was sponsored by Shire/Takeda.
Correspondence: Shannon Meeks, Aflac Cancer and Blood Disorder Center, Emory University/Children’s Healthcare of Atlanta, 2015 Uppergate Dr #442, Atlanta, GA 30032; e-mail smeeks@emory.edu; and Sean R. Stowell, Joint Program in Transfusion Medicine, Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, 630E New Research Building, Boston, MA 02115; e-mail: srstowell@bwh.harvard.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
C.M.A. and P.E.Z. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal