Choosing the best immunosuppression for acquired hemophilia A (AHA) is a narrow path between shortening the time to remission and avoiding deleterious adverse effects. The article in this issue of Blood by Simon et al1 provides some safer steps to accomplish this.
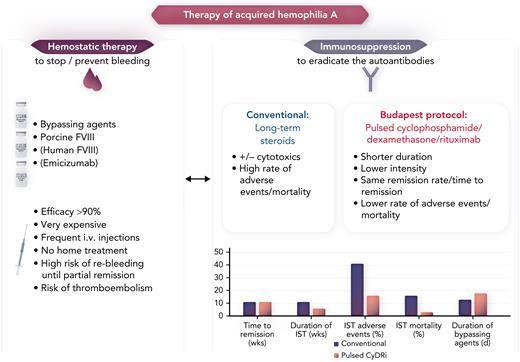
In AHA, autoantibodies block coagulation factor VIII (FVIII), which leads to severe bleeding. This requires very expensive hemostatic therapy with bypassing agents (recombinant FVIIa, activated prothrombin complex), or recombinant porcine FVIII.2 For immunosuppression (the causal therapy for eradicating the autoantibodies), daily doses of steroids with or without other cytostatic or immunosuppressive drugs, especially cyclophosphamide, for several weeks have long been used, but they cause an unacceptable high rate of adverse events (up to 46%) and mortality (16%) (see figure). These high rates have been demonstrated in the large European Acquired Haemophilia Registry (EACH2)3 and the prospective German, Austrian and Swiss Society of Thrombosis and Hemostasis Research (GTH)-AH 01/2010 trial4 in which half the affected patients were older than age 74 years and were often in frail condition.5
The figure depicts the interdependence between the expensive hemostatic therapy and the immunosuppression with a high rate of adverse events in patients with AHA. The pulsed CyDRi protocol may shift the scales, offering a safer, less toxic pathway for this largely older patient population. IST, immunosuppressive therapy. Professional illustration by Somersault18:24.
The figure depicts the interdependence between the expensive hemostatic therapy and the immunosuppression with a high rate of adverse events in patients with AHA. The pulsed CyDRi protocol may shift the scales, offering a safer, less toxic pathway for this largely older patient population. IST, immunosuppressive therapy. Professional illustration by Somersault18:24.
Simon et al report on using a different strategy for immunosuppression, which seems to be equally effective in eradicating the autoantibodies but is better tolerated with fewer adverse events. Their strategy is quite similar to some myeloma protocols: pulses of 1000 mg of cyclophosphamide (a moderate dose) on days 1 and 22 and 40 mg of dexamethasone (a high dose) plus 100 mg of rituximab (a low dose) on days 1, 8, 15, and 22 (CyDRi). This treatment is repeated, if needed, every 6 weeks until remission. This protocol has the advantage of a lower cumulative dose of cyclophosphamide and steroids and long intervals between applications, which allows the patients to recover.
Two thirds of patients needed only 1 or 2 cycles to achieve remission, but median time to remission was still as long as 77 days, during which the risk of re-bleeding was still high.6 The major advantage of this protocol was the low rate of deaths from immunosuppression or infections, although there was still a 15.6% rate of toxicity and infections. This resulted in a remarkable overall survival of 90.6% in this older-age population, much better than the rates reported by others.5 Comparing these data with the results of other large registries (see Table 3 in Simon et al) suggests a superior safety profile, but no better remission rate or resource consumption for hemostatic therapy. The median time to bleeding control was still 15 days, and half the patients needed bypassing agents for a median time of 18 days (see figure). Therefore, the effect on total treatment costs is questionable.
Clinically challenging patients with AHA were also included in this study: patients with very high titers of inhibitors and a very long time to remission, or patients with a long time to bleeding control and a need for many days of bypassing therapy. Such situations can lead to a prolonged need for immunosuppression, increased cumulative toxicity, and high consumption of resources. Even though these patients had a favorable outcome, neither the conventional nor this pulsed CyDRi protocol seem to be ideal, and the management of such patients still needs to be improved.
Nevertheless, the protocol seems feasible (although weekly visits and intravenous therapy are necessary) and safe, but more studies with larger numbers of patients are needed to further improve the management of AHA. Protocol modifications, such as reducing the steroid dose or increasing or maybe even omitting rituximab (which is still used off-label in the Simon et al study) should be evaluated. In addition, attempts to individualize immunosuppression according to factors predicting time to remission and/or adverse events (comorbidity, initial FVIII level or inhibitor titer4) could be areas for further studies.
Future strategies with low-intensity immunosuppression, based on rituximab-only under hemostatic protection with emicizumab are already under investigation7 (NCT04188639; NCT05345197). This approach may offer a less intensive, much less costly, more feasible, and safer therapeutic strategy for this rare bleeding disorder. The first data are promising and may even lead to a paradigm shift in the management of AHA. In Japan, emicizumab has already been granted regulatory approval for AHA (June 2022). For centers that cannot easily use off-label emicizumab (or rituximab), however, the pulsed CyDRi protocol (with modifications, if needed) offers an attractive alternative to conventional daily steroids or immunosuppressants.
Conflict-of-interest disclosure: P.K. has received consultancy and speaker fees, and research and travel grants from Ablynx/Sanofi, Alexion, Biotest, CSL-Behring, Novo Nordisk, Roche, and Takeda.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal