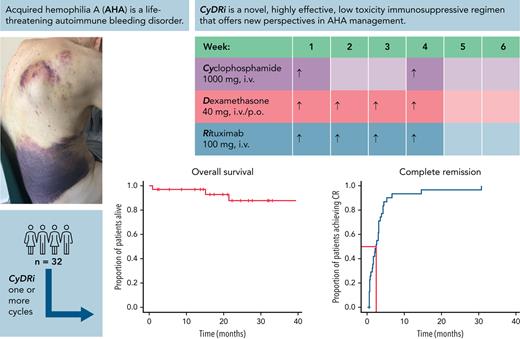
Key Points
Upfront triple combined immunosuppressive regimen (CyDRi) produces very high remission rates and survival in elderly AHA patients.
High efficacy of CyDRi is combined with low toxicity and good tolerability.
Abstract
Acquired hemophilia A (AHA) is a rare severe autoimmune bleeding disorder with significant morbidity and mortality. Although critical for disease control, there is no consensus for the best immunosuppressive regimen. Most authors use steroids first line, followed by other agents for steroid failures. Upfront combined regimens offer the advantage of reduced steroid exposure and toxicity as well as increased efficacy. We retrospectively analyzed data from 32 patients with AHA treated on an identical such institutional protocol: cyclophosphamide 1000 mg on days 1 and 22, dexamethasone 40 mg on days 1, 8, 15, and 22, and rituximab 100 mg on days 1, 8, 15, and 22 (the regimen was termed CyDRi). All patients received at least 1 cycle of CyDRi. If necessary, CyDRi was repeated until remission, no sooner than day 43 of the previous cycle. Bleeding control was rapidly achieved. The median time for bleeding control was 15.5 days (range, 0-429 days; interquartile range, 2.5-29.5 days). Thirty-one (96.8%) of 32 patients achieved durable complete remission (CR); 29 (90.6%) of 32 patients were alive at last follow-up, all of them in CR. The median time to reach first CR was 77 days (range, 19-939 days; interquartile range, 31-115 days). Toxicity and side effects were acceptable and milder than those of commonly used, prolonged steroid therapies. In conclusion, the CyDRi regimen produced markedly higher CR rates and overall survival than currently used sequential regimens. Taken together, CyDRi proved to be an attractive option for the immunosuppression of elderly patients with AHA.
Introduction
Initially called pseudohemophilia, acquired hemophilia A (AHA) has been recognized since the 1940s.1 It is a rare severe bleeding disorder caused by autoantibodies against factor VIII (FVIII) resulting in increased clearance and neutralization of FVIII. The incidence is estimated to be 1.48 cases per million.2 Bleeding manifestations are also variable, from mild to severe to life-threatening, and mortality may be as high as 22%.2-5 In 44% to 63% of patients, AHA is not associated with any medical illnesses (idiopathic AHA).2,4,6,7 In the remaining patients, AHA is associated with malignancy (6%-18%), autoimmune disorders (9%-17%), pregnancy (2%-15%), certain drugs (3%-5%), or other conditions.
The 2 pillars of treatment include acute bleeding control and immunosuppressive therapy (IST). Guidelines, based on retrospective data and expert opinion, recommend immunosuppressive treatment as soon as the diagnosis is made8,9 while also cautioning about the significant toxicity and mortality of IST in frail patients.10,11 Thus, safer IST regimens are desperately needed. However, although critical for long-term disease-free survival, these guidelines are vague on what IST is to be used. Current protocols use a sequential approach of prednisolone, 1 mg/kg per day ± cyclophosphamide, by mouth 1.5 to 2 mg/kg per day for up to 6 weeks followed by second-line agents (including cyclosporine, rituximab, and others) in case of failure. The availability of subcutaneous emicizumab (off-label in AHA) has made bleeding control easier and could theoretically allow delaying potentially toxic immunosuppression,12 and a clinical trial (#NCT04188639) is underway to test this novel approach; however, the cost of emicizumab is high, and long-term safety of delaying causative treatment is unknown. Hence, current European practice is largely in line with the recommendations for the possibly early start of immunosuppression as reflected by the European Acquired Haemophilia Registry (EACH2) international registry,13 by the Spanish Acquired Hemophilia A Spanish Registry (AHASR) registry data,14 and a German prospective observational study.15 Although similar data are not available from the United States, medical practice is safely assumed to be largely similar.
Upfront combined (“hit it hard at the beginning”) regimens offer the advantage of reduced steroid exposure and toxicity as well as increased efficacy. Therefore, in September 2009, we devised an institutional treatment protocol at St. László Campus of South-Pest Central Hospital, coined CyDRi, which uses pulse doses of cyclophosphamide, dexamethasone, and low-dose rituximab. Subsequently, all patients were treated on this protocol. Although steroids, cyclophosphamide, and rituximab are all established components of immunosuppressive regimens in AHA, CyDRi is a novel treatment concept in several ways, including: (1) the use of dexamethasone as the steroid component of the regimen; (2) the upfront combination of the 3 drugs; (3) the pulse dosing of all 3 drugs; and (4) the use of an unchanged regimen for resistant or relapsed disease. These factors make CyDRi different from all previously reported immunosuppression regimens. In September 2017, the same protocol was also introduced at Semmelweis University. The current retrospective analysis is a critical review of the first 32 consecutive patients treated with CyDRi at the 2 participating institutions.
Patients and methods
AHA was defined as low FVIII (<50 IU/dL) level and the presence of an FVIII inhibitor (>0.1 Bethesda titer unit [BU]) in a patient with any bleeding symptoms. We searched the databases of the 2 institutions after having obtained investigational review board approval for the retrospective study. We identified 32 consecutive AHA patients treated with CyDRi between the start of each institutional protocol at South-Pest Central Hospital (between October 2009 and September 2021) and at Semmelweis University (between March 2018 and September 2021). Twenty-three patients were treated on the protocol at South-Pest Central Hospital and 9 at Semmelweis University. The first enrolled patient was diagnosed in October 2009 and the last patient in July 2020. Follow-up continued through September 2021. Of note, these patients were not included in the European registry (EACH2); thus, none of them has previously been reported.
During the study period, all patients admitted with AHA to the participating institutions were treated on the protocol and were included in the study. Because relapses were treated with the same regimen, whenever it was appropriate in the analysis, we considered new cases and relapses as separate instances. With 3 relapses altogether, 35 instances were considered in some analyses. Medical history was recorded for all patients. History of autoimmune or malignant conditions was considered potentially etiologically relevant (termed: underlying disorders); all other disorders were grouped as comorbidities.
Treatment
The CyDRi regimen is a combined IST consisting of: 1000 mg cyclophosphamide IV on days 1 and 22; 40 mg dexamethasone, IV or by mouth, on days 1, 8, 15, and 22; and 100 mg rituximab, IV on days 1, 8, 15, and 22. When necessary, CyDRi was repeated no sooner than day 43 of the previous cycle. The decision to start a second or subsequent cycle of CyDRi was made based on either of the following criteria: (1) no satisfactory rise in FVIII seen by day 42 of the previous cycle (coined “slow response”); or (2) a rise in the Bethesda titer after reaching remission (coined “laboratory relapse”) detected at any time. “Slow response” is a somewhat subjective decision by the treating physician (it usually meant a plateauing FVIII level at <50 IU/dL for 1-3 weeks); if FVIII continued to rise, a watch-and-wait strategy was usually used unless there were bleeding symptoms. Detectable anti-FVIII antibodies were not formally required for a subsequent CyDRi cycle (but was >0 in 7 of 8 patients at the time of the decision to treat). Because the Bethesda assay is known to be insensitive, an FVIII level <50 IU/dL was taken as proof of the presence of antibodies whether detectable by the Bethesda assay or not.
All patients were initially treated as inpatients. Administration of bypassing agents was decided by the treating physician at the time of admission based on the presence or absence of serious active bleeding. All patients received at least 1 cycle of CyDRi. Acyclovir (400 mg once daily) viral prophylaxis was used in all patients throughout the treatment and for 6 months after the last day of the last cycle.
Laboratory tests
All laboratory tests were conducted by the individual laboratories of the 2 institutions. These laboratories, however, used identical methodologies for all assays. Factor assays were performed by using one-stage automated factor activity testing on Instrumentation Laboratory (Bedford, MA) platforms using the company’s reagents and following manufacturer instructions. Similarly, the Bethesda anti-FVIII titer determinations were performed via identical methods in the 2 laboratories using the Nijmegen modification.16 The laboratory diagnosis of AHA was established by a >0.1 BU and a low FVIII level (<50 IU/dL) combined with normal FIX, FXI, and FXII, as well as normal von Willebrand factor antigen and ristocetin cofactor activity (VWF:Ag and VWF:RCo) levels.
Outcomes assessments
Study outcomes assessed included the following: (1) complete remission (CR), which was defined by the presence of all of several criteria (cessation of bleeding, undetectable FVIII inhibitor in the Bethesda assay, restoration of FVIII activity to >50 IU/dL, and cessation of IST); (2) overall survival; (3) relapse rate; (4) remission duration; and (5) cessation of bleeding (bleeding control). Bleeding control was defined as the time point off bypassing agents after which no new clinical bleeding appeared and the patient’s hemoglobin level did not fall. If the patient was still on bypassing agents, the discontinuation of the bypassing agent was used as the first day of bleeding control. Two additional study outcomes, relapse and death rates, were assessed. Relapse was defined as the re-emergence of an FVIII inhibitor after being in CR. Patients were censored at the time of last follow-up.
Data collection and statistical analysis
Clinical and laboratory data were retrospectively collected in a standardized fashion using a case report form developed for this particular purpose.
Statistical analysis and Kaplan-Meier curves were performed by using R software. For log-rank test, a P value < 0.05 was considered statistically significant.
Results
Cohort characteristics
The study cohort’s characteristics are summarized in Table 1. Patient age varied between 53 and 87 years. Median FVIII activity at diagnosis was 1 IU/dL (range, 0-17 IU/dL; interquartile range [IQR], 0-4.3 IU/dL), and the median initial inhibitor titer was 17 BU (range, 1.3-3165 BU; IQR, 5.7-112.5 BU). Fourteen (43.8%) of the 32 patients had initial Bethesda titers >20 BU. In 13 patients (40.6%), an underlying disease was identified; the remaining 19 cases were considered idiopathic. Important clinical and laboratory data of each patient are listed in Table 1 and supplemental Table 1 (available on the Blood Web site). Median follow-up was 779.5 days (range, 27-3319 days; IQR, 455.5-1868.2 days). The median time between the first symptom and diagnosis was 14 days (range, 0-270 days; IQR, 7.5-30 days).
Demographic and clinical characteristics of the 2-institution cohort
| Characteristic . | Value . |
|---|---|
| All patients, n | 32 |
| Male | 14 (43.7) |
| Female | 18 (56.3) |
| Age, range | 53-87 |
| Mean ± SD | 74.1 ± 9.1 |
| Median (IQR) | 77 (65.8-80.3) |
| >80 y | 9 (28.1) |
| >85 y | 4 (12.5) |
| Comorbidity and underlying conditions | |
| Comorbidity index, mean ± SD | 1.6 ± 1.4 |
| ECOG performance status score, mean ± SD | 2.1 ± 0.9 |
| 4 | 3 |
| 3 | 6 |
| 2 | 13 |
| 1 | 10 |
| Underlying illnesses∗ | |
| None (idiopathic) | 19 (59.4) |
| Autoimmune | 8 (25) |
| Bullous pemphigoid | 2 |
| Graves hyperthyroidism | 2 |
| Hashimoto hypothyroidism | 2 |
| Rheumatoid arthritis | 2 |
| Systemic lupus erythematosus | 1 |
| Neoplastic | 7 (21.9) |
| Common comorbid conditions∗ | |
| Hypertension | 18 (56.3) |
| Cardiovascular disease | 10 (31.3) |
| Type 2 diabetes mellitus | 6 (18.8) |
| Chronic obstructive pulmonary disease | 6 (18.8) |
| AHA initial severity | |
| Initial FVIII, mean ± SD, IU/dL | 3 ± 4.4 |
| Median (IQR) | 1 (0-4.3) |
| Initial inhibitor, mean ± SD, BU | 177.9 ± 552.2 |
| Median (IQR) | 17 (5.7-112.5) |
| >20 BU | 14 (43.8) |
| >100 BU | 10 (31.3) |
| >1000 BU | 1 (3.1) |
| No. of CyDRi cycles needed for CR | |
| All patients, mean ± SD | 1.66 ± 1.52 |
| All patients, median (IQR) | 1 (1-2) |
| 1 cycle only | 22 |
| 2 cycles | 5 |
| 3 cycles | 4 |
| 9 cycles | 1 |
| Characteristic . | Value . |
|---|---|
| All patients, n | 32 |
| Male | 14 (43.7) |
| Female | 18 (56.3) |
| Age, range | 53-87 |
| Mean ± SD | 74.1 ± 9.1 |
| Median (IQR) | 77 (65.8-80.3) |
| >80 y | 9 (28.1) |
| >85 y | 4 (12.5) |
| Comorbidity and underlying conditions | |
| Comorbidity index, mean ± SD | 1.6 ± 1.4 |
| ECOG performance status score, mean ± SD | 2.1 ± 0.9 |
| 4 | 3 |
| 3 | 6 |
| 2 | 13 |
| 1 | 10 |
| Underlying illnesses∗ | |
| None (idiopathic) | 19 (59.4) |
| Autoimmune | 8 (25) |
| Bullous pemphigoid | 2 |
| Graves hyperthyroidism | 2 |
| Hashimoto hypothyroidism | 2 |
| Rheumatoid arthritis | 2 |
| Systemic lupus erythematosus | 1 |
| Neoplastic | 7 (21.9) |
| Common comorbid conditions∗ | |
| Hypertension | 18 (56.3) |
| Cardiovascular disease | 10 (31.3) |
| Type 2 diabetes mellitus | 6 (18.8) |
| Chronic obstructive pulmonary disease | 6 (18.8) |
| AHA initial severity | |
| Initial FVIII, mean ± SD, IU/dL | 3 ± 4.4 |
| Median (IQR) | 1 (0-4.3) |
| Initial inhibitor, mean ± SD, BU | 177.9 ± 552.2 |
| Median (IQR) | 17 (5.7-112.5) |
| >20 BU | 14 (43.8) |
| >100 BU | 10 (31.3) |
| >1000 BU | 1 (3.1) |
| No. of CyDRi cycles needed for CR | |
| All patients, mean ± SD | 1.66 ± 1.52 |
| All patients, median (IQR) | 1 (1-2) |
| 1 cycle only | 22 |
| 2 cycles | 5 |
| 3 cycles | 4 |
| 9 cycles | 1 |
Data are expressed as n (%) unless otherwise indicated.
Numbers are not additive because overlaps occurred (eg, patient 3B#4 had RA and pemphigoid). Details are provided in supplemental Table 1.
Three relapses of 2 patients (LA#7 and LA#13)
| Patient No. . | Line . | FVIII at diagnosis (IU/dL) . | BU at diagnosis . | CyDRi (no. of cycles) . | TTBC (d) . | Days on bypass . | TTCR (d) . | CR duration (d) . |
|---|---|---|---|---|---|---|---|---|
| LA#7 | 1 | 5 | 8.5 | 1 | 45 | 45 | 92 | 357 |
| LA#7 | 2 | 11 | NA | 1 | 0 | 0 | 22 | 1385 |
| LA#7 | 3 | 10 | 0.9 | 1 | 0 | 0 | 99 | 1372 |
| LA#13 | 1 | 1 | 140 | 2 | 8 | 3 | 67 | 160 |
| LA#13 | 2 | 19 | 0 | 1 | 0 | 0 | 28 | 161 |
| Patient No. . | Line . | FVIII at diagnosis (IU/dL) . | BU at diagnosis . | CyDRi (no. of cycles) . | TTBC (d) . | Days on bypass . | TTCR (d) . | CR duration (d) . |
|---|---|---|---|---|---|---|---|---|
| LA#7 | 1 | 5 | 8.5 | 1 | 45 | 45 | 92 | 357 |
| LA#7 | 2 | 11 | NA | 1 | 0 | 0 | 22 | 1385 |
| LA#7 | 3 | 10 | 0.9 | 1 | 0 | 0 | 99 | 1372 |
| LA#13 | 1 | 1 | 140 | 2 | 8 | 3 | 67 | 160 |
| LA#13 | 2 | 19 | 0 | 1 | 0 | 0 | 28 | 161 |
TTBC, time to bleeding control.
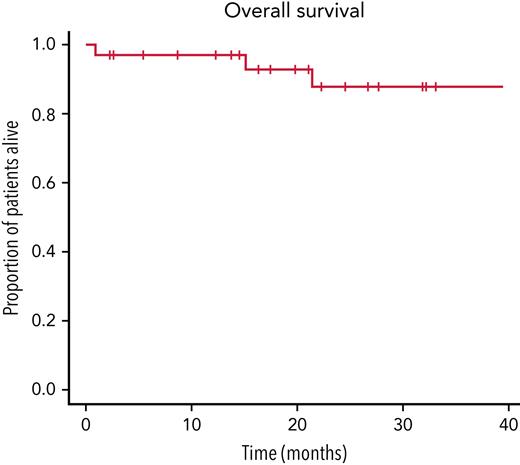
Overall survival of the cohort treated with CyDRi. Kaplan-Meier curve was plotted for the entire cohort (N = 32). Median follow-up was 779.5 (range, 27-3319; IQR, 455.5-1868.2) days. For each patient, day 1 was the day of first admission, which was typically also day 1 of the first cycle of CyDRi.
Overall survival of the cohort treated with CyDRi. Kaplan-Meier curve was plotted for the entire cohort (N = 32). Median follow-up was 779.5 (range, 27-3319; IQR, 455.5-1868.2) days. For each patient, day 1 was the day of first admission, which was typically also day 1 of the first cycle of CyDRi.
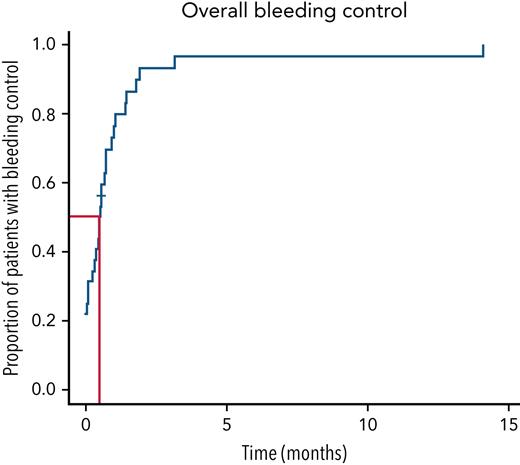
Bleeding control in the cohort treated with CyDRi. Kaplan-Meier curve was plotted for the entire cohort (N = 32). Median time to bleeding control was 15.5 (range, 0-429; IQR, 2.5-29.5) days. Median follow-up for the cohort was 779.5 (range, 27-3319; IQR, 455.5-1868.2) days.
Bleeding control in the cohort treated with CyDRi. Kaplan-Meier curve was plotted for the entire cohort (N = 32). Median time to bleeding control was 15.5 (range, 0-429; IQR, 2.5-29.5) days. Median follow-up for the cohort was 779.5 (range, 27-3319; IQR, 455.5-1868.2) days.
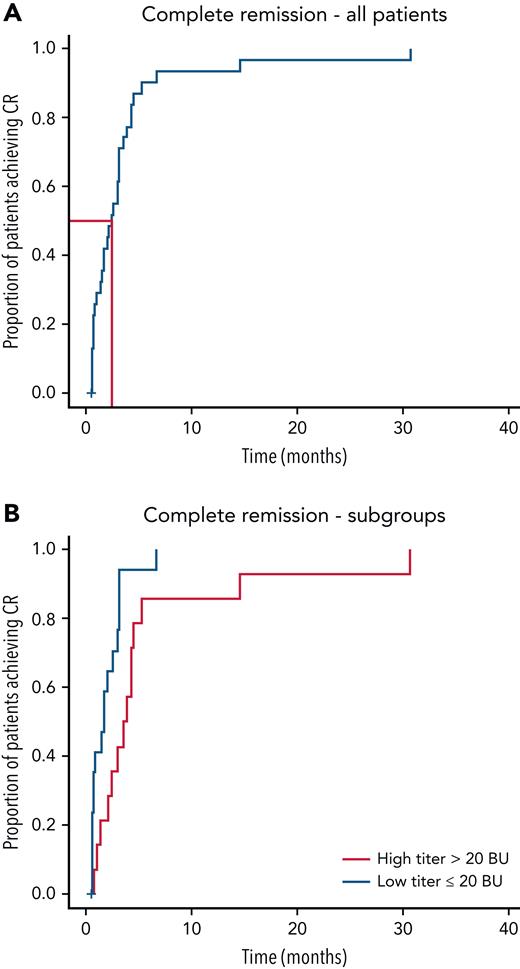
First CR. (A) Kaplan-Meier curves were plotted for the entire cohort (N = 32). TTCR was median 77 (range, 19-939; IQR, 31-115) days. There were 31 remission events in the cohort (96.9%). (B) Kaplan-Meier curves for CR were plotted for 2 subgroups: patients with high titer (>20 BU, 14 patients) and low titer (≤20 BU, 18 patients) anti-FVIII antibodies (P = .009). Median follow-up for the cohort was 779.5 (range, 27-3319; IQR, 455.5-1868.2) days.
First CR. (A) Kaplan-Meier curves were plotted for the entire cohort (N = 32). TTCR was median 77 (range, 19-939; IQR, 31-115) days. There were 31 remission events in the cohort (96.9%). (B) Kaplan-Meier curves for CR were plotted for 2 subgroups: patients with high titer (>20 BU, 14 patients) and low titer (≤20 BU, 18 patients) anti-FVIII antibodies (P = .009). Median follow-up for the cohort was 779.5 (range, 27-3319; IQR, 455.5-1868.2) days.
First CR duration. For this analysis, Kaplan-Meier curves were plotted from time 0, the time when patients achieved a first CR. Relapses were not considered as separate events in this analysis (n = 31 CR events). (A) CR duration of the entire cohort (n = 31). (B) In the comparison of 2 subgroups (patients with high [>2] and low [≤2] comorbidity index), there were 8 and 23 remission events, respectively (P = .002). Median observation time after first CR was a median of 630 (range, 17-3178; IQR, 280.5-1577.0) days. For the 3 relapses, subsequent CR durations ranged from 161 to 1385 days (median, 1372 days).
First CR duration. For this analysis, Kaplan-Meier curves were plotted from time 0, the time when patients achieved a first CR. Relapses were not considered as separate events in this analysis (n = 31 CR events). (A) CR duration of the entire cohort (n = 31). (B) In the comparison of 2 subgroups (patients with high [>2] and low [≤2] comorbidity index), there were 8 and 23 remission events, respectively (P = .002). Median observation time after first CR was a median of 630 (range, 17-3178; IQR, 280.5-1577.0) days. For the 3 relapses, subsequent CR durations ranged from 161 to 1385 days (median, 1372 days).
Treatment efficacy
Survival (Figure 1): Twenty-nine (90.6%) of 32 patients were alive, all of them in durable CR, at last follow-up (median, 779.5 days; range, 27-3319 days; IQR, 455.5-1868.2 days). Three patients died during the observation period. One elderly, very frail patient with a femoral neck fracture died of hypostatic pneumonia 27 days after diagnosis, where we could not exclude a contribution from the IST. Another patient, who had been in CR for >1 year, died of a septic episode, which was linked to his underlying condition (systemic lupus erythematosus, lupus nephritis, renal failure requiring dialysis, and poorly controlled diabetes mellitus). The third patient died of independent comorbidity (hypertensive cardiomyopathy and severe heart failure) >1.5 years after achieving durable CR. He was closely monitored with several hospitalizations in his last months, and remained in remission, and had no bleeding complications in spite of anticoagulation therapy (for atrial fibrillation and dilated cardiomyopathy). Overall survival showed no correlation with: (1) initial inhibitor titer (P = .1); (2) initial FVIII activity (P = .3); (3) Eastern Cooperative Oncology Group (ECOG) status (P = .7); or (4) comorbidity index (P = .055).
Bleeding control (Figure 2): Twelve (37.5%) of 32 patients presented with active bleeding symptoms requiring bypassing agents (simultaneously with the CyDRi regimen) from the time of admission until bleeding control. The remaining 20 patients had a recent history of bleeding but were not actively bleeding at the time of admission and were given CyDRi without concurrent bypassing agents; 3 of them had a subsequent episode of bleeding requiring one of the bypassing agents. Although 17 patients did not bleed after admission, given the strict definition of bleeding control (no bleeding off bypassing agents and stable hemoglobin), the time to bleeding control was 0 days only in 7 patients. The other 10 patients were either on a bypassing agent as a precaution after a recent bleed before admission, or stable hemoglobin was not immediately established for an actual fall or lack of data. The median time needed to achieve bleeding control in the whole cohort was 15.5 days (range, 0-429 days; IQR, 2.5-29.5 days). By the end of the second month, the majority (29 of 32) of the patients were free of bleeding. No significant correlation was found between the time to achieve bleeding control and the initial inhibitor titer (P = 1), FVIII activity (P = .6), ECOG performance status (P = .2), or comorbidity index (P = .3). Days on bypassing agents: In case of bleeding, patients were given a bypassing agent at the discretion of the treating physician. Fifteen patients (46.9%) received some bypassing agent at least once during the observation period. The median number of days spent on any bypassing agent was 18 (range, 3-159 days; IQR, 15-27.3 days). Eight patients received recombinant FVIIa, 5 patients received anti-inhibitor coagulant complex APCC, and 2 patients who initially received recombinant FVIIa were later switched to APCC. The reason to switch was ease of administration as outpatient.
CR (Figure 3A): CR was achieved in 31 (96.9%) of 32 patients at last follow-up. CR was not achieved in 1 elderly patient, who died of hypostatic pneumonia before achieving CR. Her inhibitor titer was falling at the time of her death. The median time to reach CR (TTCR) was 77 days (range, 19-939 days; IQR, 31-115 days). One of the patients had exceptionally high Bethesda titer (3165 BU) at the beginning. She needed 939 days to achieve CR. Statistically significant correlation was found between TTCR and initial inhibitor concentration (P = .009) (Figure 3B). There was no correlation between TTCR and initial FVIII activity (P = .09), ECOG status (P = .4), or comorbidity index (P = .6).
First CR duration (Figure 4A): The median duration of first CR in this cohort was 630 days (range, 17-3178 days; IQR, 280.5-1577.0 days) during the observation period. Correlation was observed between CR duration and comorbidity index (P = .002) (Figure 4B). This was mainly due to the higher tendency to relapse of patients with higher comorbidity index. However, no significant correlation was found between CR duration and initial inhibitor titer (P = .9), initial FVIII activity (P = .7), or ECOG status (P = .4).
Relapses and subsequent CR duration: Only 2 of the 31 patients in CR experienced a relapse (relapse rate, 6.5%). These 2 patients (#LA7 and #LA13) had a total of 3 relapse episodes. The 5 episodes of AHA of these 2 patients are outlined in Table 2. The CyDRi regimen proved equally efficacious in treating relapses as it was in first line. Time to reach a second CR was 22, 91, and 28 days, respectively. The duration of the subsequent CRs for the 3 relapses ranged from 161 to 1385 days (median, 1372 days).
Number of CyDRi cycles needed: Ten (31.3%) of 32 patients required >1 cycle of CyDRi to achieve a durable CR, either due to slow response (8 patients) or to a laboratory relapse (2 patients). Four patients required 3 cycles, and 1 patient, whose Bethesda titer at the beginning was exceptionally high (3165 BU), received 9 cycles of CyDRi. The remaining 5 patients received 2 cycles. CyDRi seemed equally effective in treating relapses. Two of 31 patients in CR had a relapse after a first CR, whereas 29 patients (93.5%) reached permanent CR at first CR. Of the 2 relapsing patients, #LA13 had one laboratory relapse (after which TTCR was 28 days), and #LA7 had two. After the first relapse, TTCR was 22 days and after the second episode it was 91 days. No bleeding was observed during laboratory relapses, and only one cycle of CyDRi was needed to reach CR again in all 3 relapse episodes. Patient 3B#3 presented with extremely high (>3000 BU) Bethesda titers after a hip replacement surgery (Table 1; supplemental Table 1). CyDRi was started, and her titers decreased gradually with each of the subsequent eight CyDRi cycles over a period of 14 months, but significant residual (1-4 BU) inhibitors with undetectable FVIII remained. At that point, the COVID-19 epidemic kept the patient from following up in the clinic, and she did not receive any treatment for 16 months. Once she returned to the clinic, she was found to have a <1 BU inhibitor titer with measurable (23 IU/dL) FVIII level for the first time. At that point, the patient received a ninth CyDRi cycle and went into CR within 4 weeks (FVIII 64 IU/dL). She had no significant toxicity from CyDRi.
Treatment toxicity
Side effects were acceptable and seemed fewer compared with commonly used prolonged steroid therapies: one patient acquired pneumonia 3 weeks after discharge from the hospital, and another elderly, frail patient with hip fracture died of a hypostatic pneumonia. One patient developed Clostridium difficile colitis during hospitalization, and one patient had Klebsiella cystitis. All infections responded to antibiotic treatment. The only severe infectious complication was the development of a retroperitoneal abscess at the site of a former hematoma several weeks after the cessation of bleeding and having achieved CR. This complication required surgery, which proved successful. Of note, cyclophosphamide was only associated with short, mild cytopenia, and was not complicated by febrile neutropenia in any of the patients. One elderly patient was given granulocyte colony-stimulating factor to shorten neutropenia. Neither steroid-induced diabetes nor psychiatric complications were observed.
Discussion
We report a 2-institution experience with treating AHA patients with the CyDRi IST regimen, which we found surprisingly effective and well tolerated compared with published cohorts of sequential regimens. Comparison of cohorts published in different settings is inherently difficult. Comparing our cohort vs the published international EACH2,7,13,17 the German GTH-AH 01/2010,15,18 the UK2 survey about AHA, and vs data from 2 more recently published registries (the Spanish AHASR14 and the Chinese CARE [China Acquired Hemophilia Registry]19) registries is further impeded by the fact that data collection in these studies was vastly different. The UK study is a survey sent to all UK Haemophilia Centres, the EACH2 is an international registry with voluntary data entry, the Spanish and Chinese studies obtain data from nationally organized registries, and the German study is a prospective observational study using a uniform treatment regimen. Our current study is a retrospective analysis of 32 consecutive patients at 2 institutions using the CyDRi IST regimen. We nevertheless attempted to extract data from these reports comparable to our own (summarized in Table 3). To decrease the chances of bias, we first compared cohort characteristics to make sure any advantageous differences were not due to selection of a lower risk patient population. Median patient age, gender distribution, and comorbidities in our cohort are similar to the ones reported in the 5 cohorts. The older patient population reported from the United Kingdom probably reflects differences in the age trees of the general populations in Hungary and the United Kingdom. The proportion of patients with high (>20 BU) or very high (>100 BU) inhibitors is actually higher in our cohort, supporting the notion that low-risk patient selection is not responsible for the superior outcome. Follow-up is longer in our cohort, which allowed the capture of any possible late complications or relapses.
Comparison of key characteristics of the current cohort vs those of 5 recently published cohorts in the literature (data were extracted from the articles shown)
| Cohort characteristics . | Current study . | EACH27,13 . | German15,18 . | United Kingdom2,22 . | Spanish AHASR14 . | Chinese CARE19 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 32 | 501 | 102 | 172 | 151 | 187 | ||||||||
| Age, median, y | 77 | 73.9 | 74 | 78 | 74 | 52 | ||||||||
| Age >80 y, % | 28.1 | 19.1 (non-peripartum) | NA | NA | 28.6 | 5.5 (non-peripartum) | ||||||||
| Age >85 y, % | 12.5 | 7.9 (non-peripartum) | NA | 22.5 (non-peripartum) | NA | 1.8 (non-peripartum) | ||||||||
| Percent female | 56.3 | 50.7 | 51.8 | 43 | 57.4 | 43.7 | 54.5 | |||||||
| Initial FVIII, median, IU/dL | 1.0 | 2 | 1.4 | 5 | 2 | 1.7 | 1.7 | |||||||
| Initial inhibitor, median, BU | 17 | 12.8 | 19 | 8 | 18 | 13 | 13 | |||||||
| High inhibitor (>20 BU) | 14/32 (43.8%) | NA | 40/102 (39.2%) | NA | NA | NA | ||||||||
| Very high inhibitor (>100 BU) | 10/32 (31.3%) | 62/501 (12.4%) | 14/102 (13.7%) | 16/172 (9.3%) | 15/145 (10.3%) | 18/187 (9.6%) | ||||||||
| Extreme inhibitor (>1000 BU) | 1/32 (3.1%) | 0/501 (0%) | 1/102 (1.0%) | 0/172 (0%) | 4/145 (2.8%) | 1/187 (0.5%) | ||||||||
| Idiopathic (%) | 59.4 | 61 | 68 | 67 | 63.3 | 44.1 | 54.4 | |||||||
| Autoimmune (%) | 25 | 16 | 17 | 20 | 16.7 | 31.7 | 12.5 | |||||||
| Malignancy (%) | 21.9 | 11 | 10 | 13 | 14.7 | 10.3 | 6 | |||||||
| Pregnancy (%) | 0 | 12 | 2 | 5 | 2 | 6.2 | 12.5 | |||||||
| Median follow-up, d | 779.5 | 262 | 262 | NA (survey for patients in 2 y) | 360 | 205 | ||||||||
| Treatment | CyDRi | P | P + by mouth Cy | P ± by mouth Cy ± R | Sequential P/Cy | Simultaneous P + Cy | P | P + by mouth Cy | P + CNI | R ± | P | P + Cy | R + | Other |
| Alive in CR∗ at last F/U | 29/32 (90.6%) | 90/142 (63.4%) | 43/83 (62.3%) | 49/102 (48%) | 78/172 (45.3%) | 100/151 (66.2%) | 115/155 (74.2%) | |||||||
| TTCR, median, d | 77 | 108 | 74 | 79 | 49 | 39 | 30 | 53 | 45 | 42 | 69 | 62 | 47 | 74 |
| Alive, no CR,∗ last F/U | 0/32 (0%) | 5/142 (3.5%) | 1/83 (1.2%) | 19/102 (18.6%) | 8/172 (4.7%) | 15/151 (9.9%) | 29/155 (18.7%) | |||||||
| Lost to F/U, not accounted for | 0 | 7/142 (4.9%) | 14/83 (16.9%) | 0 | 16/172 (9.3%) | 0 | 22 (11.7%) | |||||||
| Relapse rate (% from CR∗) | 2/31 (6.5%) | 15/83 (18.1%) | 8/66 (12.1%) | 15/62 (24.2%) | 18/90 (20%) | 8/112 (7.1%) | 13/155 (8.4%) | |||||||
| All-cause mortality last F/U | 3/32 (9.4%) | 40/142 (28.2%) | 25/83 (30.1%) | 34/102 (33.3%) | 55/127 (43.3%) | 18/48 (37.5%) | 36/151 (23.8%) | 11/165 (6.7%) | ||||||
| Bleeding-related mortality | 0/32 (0%) | 1/142 (0.7%) | 0/83 (0%) | 3/102 (2.9%) | 13/175 (7.4%) | 5/151 (3.3%) | 6/165 (0.6%) | |||||||
| Treatment-related mortality | 1/32 (3.1%) | 5/142 (3.5%) | 4/83 (4.8%) | 16/102 (15.7%) | 12/175 (6.9%) | 15/151 (9.9%) | 2/165 (1.2%) | |||||||
| Mortality related to underlying disease | 1/32 (3.1%) | 6/142 (4.2%) | 4/83 (4.8%) | 3/102 (2.9%) | NA | 16/151 (10.6%) | 2/165 (1.2%) | |||||||
| Toxicity (AE) | 5/32 (15.6%) | 36/142 (25%) | 34/83 (41%) | 67/102 (66%) | 57/112 (51%) | NA | 11/155 (7.1%) | |||||||
| Infection | 5/32 (15.6%) | 23/142 (16%) | 22/83 (27%) | 37/102 (36%) | 37/112 (33%) | NA | 4/155 (2.6%) | |||||||
| Symptomatic neutropenia | 0 | 2/142 (1%) | 12/83 (14%) | 1/102 (1%) | 13/112 (12%) | NA | 1/155 (0.6%) | |||||||
| Diabetes mellitus | 0 | 11/142 (8%) | 5/83 (6%) | 12/102 (12%) | 9/112 (8%) | NA | NA | |||||||
| Psychiatric | 0 | 6/142 (4%) | 3/83 (4%) | 3/102 (3%) | 2/112 (2%) | NA | NA | |||||||
| Thromboembolic/cardiovascular | 0 | 10/501 (2%) | 8/102 (7.8%) | 0 | NA | 3/155 (1.9%) | ||||||||
| Those with no AE | 27/32 (84.4%) | 106/142 (75%) | 49/83 (59%) | 35/102 (34%) | 55/112 (49%) | NA | 144/155 (92.9%) | |||||||
| Cohort characteristics . | Current study . | EACH27,13 . | German15,18 . | United Kingdom2,22 . | Spanish AHASR14 . | Chinese CARE19 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | 32 | 501 | 102 | 172 | 151 | 187 | ||||||||
| Age, median, y | 77 | 73.9 | 74 | 78 | 74 | 52 | ||||||||
| Age >80 y, % | 28.1 | 19.1 (non-peripartum) | NA | NA | 28.6 | 5.5 (non-peripartum) | ||||||||
| Age >85 y, % | 12.5 | 7.9 (non-peripartum) | NA | 22.5 (non-peripartum) | NA | 1.8 (non-peripartum) | ||||||||
| Percent female | 56.3 | 50.7 | 51.8 | 43 | 57.4 | 43.7 | 54.5 | |||||||
| Initial FVIII, median, IU/dL | 1.0 | 2 | 1.4 | 5 | 2 | 1.7 | 1.7 | |||||||
| Initial inhibitor, median, BU | 17 | 12.8 | 19 | 8 | 18 | 13 | 13 | |||||||
| High inhibitor (>20 BU) | 14/32 (43.8%) | NA | 40/102 (39.2%) | NA | NA | NA | ||||||||
| Very high inhibitor (>100 BU) | 10/32 (31.3%) | 62/501 (12.4%) | 14/102 (13.7%) | 16/172 (9.3%) | 15/145 (10.3%) | 18/187 (9.6%) | ||||||||
| Extreme inhibitor (>1000 BU) | 1/32 (3.1%) | 0/501 (0%) | 1/102 (1.0%) | 0/172 (0%) | 4/145 (2.8%) | 1/187 (0.5%) | ||||||||
| Idiopathic (%) | 59.4 | 61 | 68 | 67 | 63.3 | 44.1 | 54.4 | |||||||
| Autoimmune (%) | 25 | 16 | 17 | 20 | 16.7 | 31.7 | 12.5 | |||||||
| Malignancy (%) | 21.9 | 11 | 10 | 13 | 14.7 | 10.3 | 6 | |||||||
| Pregnancy (%) | 0 | 12 | 2 | 5 | 2 | 6.2 | 12.5 | |||||||
| Median follow-up, d | 779.5 | 262 | 262 | NA (survey for patients in 2 y) | 360 | 205 | ||||||||
| Treatment | CyDRi | P | P + by mouth Cy | P ± by mouth Cy ± R | Sequential P/Cy | Simultaneous P + Cy | P | P + by mouth Cy | P + CNI | R ± | P | P + Cy | R + | Other |
| Alive in CR∗ at last F/U | 29/32 (90.6%) | 90/142 (63.4%) | 43/83 (62.3%) | 49/102 (48%) | 78/172 (45.3%) | 100/151 (66.2%) | 115/155 (74.2%) | |||||||
| TTCR, median, d | 77 | 108 | 74 | 79 | 49 | 39 | 30 | 53 | 45 | 42 | 69 | 62 | 47 | 74 |
| Alive, no CR,∗ last F/U | 0/32 (0%) | 5/142 (3.5%) | 1/83 (1.2%) | 19/102 (18.6%) | 8/172 (4.7%) | 15/151 (9.9%) | 29/155 (18.7%) | |||||||
| Lost to F/U, not accounted for | 0 | 7/142 (4.9%) | 14/83 (16.9%) | 0 | 16/172 (9.3%) | 0 | 22 (11.7%) | |||||||
| Relapse rate (% from CR∗) | 2/31 (6.5%) | 15/83 (18.1%) | 8/66 (12.1%) | 15/62 (24.2%) | 18/90 (20%) | 8/112 (7.1%) | 13/155 (8.4%) | |||||||
| All-cause mortality last F/U | 3/32 (9.4%) | 40/142 (28.2%) | 25/83 (30.1%) | 34/102 (33.3%) | 55/127 (43.3%) | 18/48 (37.5%) | 36/151 (23.8%) | 11/165 (6.7%) | ||||||
| Bleeding-related mortality | 0/32 (0%) | 1/142 (0.7%) | 0/83 (0%) | 3/102 (2.9%) | 13/175 (7.4%) | 5/151 (3.3%) | 6/165 (0.6%) | |||||||
| Treatment-related mortality | 1/32 (3.1%) | 5/142 (3.5%) | 4/83 (4.8%) | 16/102 (15.7%) | 12/175 (6.9%) | 15/151 (9.9%) | 2/165 (1.2%) | |||||||
| Mortality related to underlying disease | 1/32 (3.1%) | 6/142 (4.2%) | 4/83 (4.8%) | 3/102 (2.9%) | NA | 16/151 (10.6%) | 2/165 (1.2%) | |||||||
| Toxicity (AE) | 5/32 (15.6%) | 36/142 (25%) | 34/83 (41%) | 67/102 (66%) | 57/112 (51%) | NA | 11/155 (7.1%) | |||||||
| Infection | 5/32 (15.6%) | 23/142 (16%) | 22/83 (27%) | 37/102 (36%) | 37/112 (33%) | NA | 4/155 (2.6%) | |||||||
| Symptomatic neutropenia | 0 | 2/142 (1%) | 12/83 (14%) | 1/102 (1%) | 13/112 (12%) | NA | 1/155 (0.6%) | |||||||
| Diabetes mellitus | 0 | 11/142 (8%) | 5/83 (6%) | 12/102 (12%) | 9/112 (8%) | NA | NA | |||||||
| Psychiatric | 0 | 6/142 (4%) | 3/83 (4%) | 3/102 (3%) | 2/112 (2%) | NA | NA | |||||||
| Thromboembolic/cardiovascular | 0 | 10/501 (2%) | 8/102 (7.8%) | 0 | NA | 3/155 (1.9%) | ||||||||
| Those with no AE | 27/32 (84.4%) | 106/142 (75%) | 49/83 (59%) | 35/102 (34%) | 55/112 (49%) | NA | 144/155 (92.9%) | |||||||
AE, adverse event; CNI, calcineurin inhibitors; Cy, cyclophosphamide; F/U, follow-up; NA, not available; P, prednisone; R, rituximab.
CR considered as no bleed, no inhibitor, FVIII >50 IU/dL, and immunosuppression stopped.
Treatment efficacy is reflected by the CR rate, which seems to be markedly higher in our cohort compared with published data: 31 (96.9%) of the 32 current study patients achieved durable CR and, at last follow-up, 29 (90.6%) of the 32 patients were alive, all in CR, compared with 45% to 75% in the published cohorts (Table 3). CyDRi takes a novel approach to IST in several ways, which could theoretically individually or in combination result in the observed difference in outcomes: (1) it uses dexamethasone as the steroid component of the regimen; (2) it combines 3 drugs upfront; and (3) it uses pulse dosing of all 3 drugs. In addition, the CyDRi IST uses an unchanged regimen for resistant or relapsed disease. The median TTCR was 77 days in our cohort, which seems to be similar or shorter than that reported by other investigators, and is mainly influenced by the initial inhibitor titer in that a higher concentration of antibodies takes longer to clear (Figure 3B). The relapse rate (6.3%) after CyDRi is similar or lower than that observed in other studies (7%-24%) (Table 3). CR duration is influenced by mortality and relapses. Unexpectedly, we found a correlation between CR duration and the comorbidity index. Patients with higher comorbidity had a higher tendency for relapse. Although this difference is statistically significant, the confidence in the correlation between relapse and comorbidity is low, as only 2 patients relapsed in our cohort.
A key issue in AHA management is bleeding control, which is a combined result of immunosuppression and hemostatic therapy. Comparing our experience vs reported data is hampered by the fact that detailed timing of bleeding events is only provided in the GTH-AH study, and differences in the reported parameters make a direct comparison impossible. In any case, bleeding is rapidly controlled after starting CyDRi in most patients. Median time of bleeding control was 15.5 days, and although there were a few slow responders, most of the bleeds (28 of 32) were durably controlled within 2 months (Figure 2). Moreover, bleeding-related mortality was 0% in our cohort, whereas all compared studies report fatal bleeding events (Table 3). Finally, in line with all previous investigators, we found that the main risk factor for bleeding is an FVIII level <50 IU/dL, but below that level, the risk of bleeding is significant regardless of the actual FVIII, age, ECOG status, or comorbidity index.
Treatment-related toxicity is another key issue in AHA management, as all groups report a higher morbidity and mortality related to the IST than to bleeding itself. Thus, the advantageous toxicity profile of CyDRi is a major determinant of the superior outcome observed (Table 3), and it may eventually result in a shift in guidelines, which currently caution about the toxicity of immunosuppression,10,11 to recommending causative treatment to more patients.
Overall survival is a hard end point and reflects both treatment efficacy and toxicity. Although survival in AHA is heavily influenced by patient age and comorbidity, median age and the proportion of patients aged >85 years are comparable to other published AHA populations (Table 3) or reflect population differences as discussed earlier. Comorbidity is also difficult to compare but seems comparable to other studies. The proportion of patients with an active malignancy is not reported in the literature, and therefore comparison is impossible. Patients with an active malignancy were not present in our cohort (ie, all patients were in CR without antineoplastic treatment). In any case, the main reason for the different outcomes seems to be differences in the treatment regimens.
Obviously, the current study has several limitations. First, it was a retrospective study. However, the shortcomings of retrospective data analysis are offset by the fact that patients were treated on a uniform institutional protocol, which was prospectively laid out in September 2009. In addition, we captured all consecutive patients admitted with the diagnosis of AHA during the study period. Second, the number of patients in our study (N = 32) is lower than that of nationwide or international studies. This is necessarily due to the rarity of AHA. However, we feel that the uniformity of the treatment efficacy in our cohort makes it a valid comparison. Also, our study seems representative in Hungary. If one considers that Hungary is a country of 10 million, and the likely incidence of AHA in Europe is 1.48 per million,2 then during the study period (12 years), 177 to 178 new cases of AHA were expected, of which our cohort captured 32 patients (18%). In comparison, in the EACH2 registry,7 the largest collection of patients with AHA, the median recruitment rate for each country was 12.1%. Our cohort is also fraught with the possibility of referral bias. However, both institutions are considered leading academic institutions in their areas of referral, and such bias would likely select for more severe cases. Indeed, some patients were transferred from other academic institutions for treatment. Finally, outcome definitions vary from study to study, making comparisons difficult. For example, investigators have been wrestling how to best quantitate location-specific bleeding complications in AHA and how to measure the efficacy of hemostatic treatment.20 Here, we used a different approach believed to have clinical relevance: the time to bleeding control. Although this parameter captures an important clinical feature, it does have a subjective element (ie, the decision when to stop hemostatic treatment). Although we believe that the practice of using hemostatic therapy is consistently restrictive within our study, we must acknowledge that practices may be significantly different between countries with varying resources and reimbursement settings.
The relatively small number of patients in the current study does not allow for an independent evaluation of risk factors associated with poor outcome. Age and comorbidity (other than pregnancy) has consistently been associated with poor outcome in other populations. In accordance with the emerging picture from the literature, our only patient who died before achieving a complete response was the second oldest patient in our group (84 years of age), and the 2 other patients who died during the follow-up period had severe comorbidities. In addition to well-known autoimmune disorders, we noted 2 cases in our cohort with association to bullous pemphigoid, emerging as a novel disease association.21
In summary, we report that the upfront combined regimen, coined CyDRi, was found to be rapidly effective in an elderly AHA patient population with remarkably low toxicity and good overall survival. Whether all components of the regimen are necessary for the superior outcome could only be answered in a well-designed prospective clinical trial. At this time, however, CyDRi should be considered an attractive option for the immunosuppression of elderly patients with AHA.
Note added in proof
Although their data were not available for detailed analysis, an additional 5 patients with AHA were treated by authors M.G. and I.B. with the same treatment protocol at Emory University between 2015 and 2017. All 5 entered CR without major toxicity.
Acknowledgments
The authors thank Martha Arellano, Ginny Merrill, and Michael Brown for their insightful comments and discussions as well as invaluable technical help.
This work was partially supported by the Hungarian National Research Development and Innovation Office (NFKI) grant OTKA-K19_131945 (I.B.).
Authorship
Contribution: B.S. wrote the paper and performed the analysis; A.C. wrote the paper and participated in trial design; J.D., P.F., L.H., R.H., L.M., T.M., A.S, and N.W. contributed to the study design and analysis of clinical data; A.K. provided expert help on all statistical analyses; M.G. participated in trial design and paper revisions; and I.B. devised the treatment protocol, designed and oversaw the study, and was responsible for paper revisions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Imre Bodó, Department of Internal Medicine and Hematology, Semmelweis University, 46 Szentkirályi u., Budapest, 1088, Hungary; e-mail: bodoimre.md@gmail.com.
References
Author notes
∗B.S. and A.C. contributed equally to the manuscript.
Requests for original data may be submitted to the corresponding author (e-mail: bodoimre.md@gmail.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![First CR duration. For this analysis, Kaplan-Meier curves were plotted from time 0, the time when patients achieved a first CR. Relapses were not considered as separate events in this analysis (n = 31 CR events). (A) CR duration of the entire cohort (n = 31). (B) In the comparison of 2 subgroups (patients with high [>2] and low [≤2] comorbidity index), there were 8 and 23 remission events, respectively (P = .002). Median observation time after first CR was a median of 630 (range, 17-3178; IQR, 280.5-1577.0) days. For the 3 relapses, subsequent CR durations ranged from 161 to 1385 days (median, 1372 days).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/18/10.1182_blood.2022016873/6/m_blood_bld-2022-016873-gr4.jpeg?Expires=1769080249&Signature=3MPs20fVPqGFfk-7N1CTOmE62ks3QvanCpijWXqTINYMLpbJdjxJdngGKbNZ6pPCzNXnChN2XoS6ebfXMMGqcflpm4rR13TkaHvVo85tsMSiGZs0k1pl1rdWkIuavm3TJpG3DQT89ITfCtq4Zt5FdRUKN58NT~DAj9FYGxT0XjG0gd0w4FGSeKC9iO0yF4B8DVNLabFHk51IR0sM7iqaHkbpjfujP25M2-PG1K5G~HJlWWtl0qPr3b6pVD7AVPP6DTZktM2eeinfzXAfTA9xyTgFTmVj-FvwUSmfNsd2NQrY-eWIB96CzekfayMdEy1E8WkV2WN7UqFfB5c1xfhU2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal