Key Points
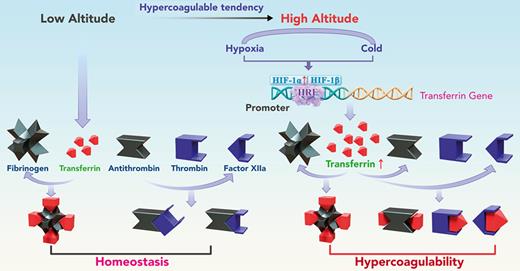
Harmful environmental factors at high altitude upregulate transferrin, which induces hypercoagulability and thromboembolic events.
Transferrin interference may provide a promising strategy for the treatment of high altitude–induced thromboembolic disorders.
Abstract
Studies have shown significantly increased thromboembolic events at high altitude. We recently reported that transferrin could potentiate blood coagulation, but the underlying mechanism for high altitude–related thromboembolism is still poorly understood. Here, we examined the activity and concentration of plasma coagulation factors and transferrin in plasma collected from long-term human residents and short-stay mice exposed to varying altitudes. We found that the activities of thrombin and factor XIIa (FXIIa) along with the concentrations of transferrin were significantly increased in the plasma of humans and mice at high altitudes. Furthermore, both hypoxia (6% O2) and low temperature (0°C), 2 critical high-altitude factors, enhanced hypoxia-inducible factor 1α (HIF-1α) levels to promote the expression of the transferrin gene, whose enhancer region contains HIF-1α binding site, and consequently, to induce hypercoagulability by potentiating thrombin and FXIIa. Importantly, thromboembolic disorders and pathological insults in mouse models induced by both hypoxia and low temperature were ameliorated by transferrin interferences, including transferrin antibody treatment, transferrin downregulation, and the administration of our designed peptides that inhibit the potentiation of transferrin on thrombin and FXIIa. Thus, low temperature and hypoxia upregulated transferrin expression–promoted hypercoagulability. Our data suggest that targeting the transferrin-coagulation pathway is a novel and potentially powerful strategy against thromboembolic events caused by harmful environmental factors under high-altitude conditions.
Introduction
Life at high altitude is physiologically challenging for animals. There are 3 major high-altitude regions in the world, including the Himalayas (average altitude, 4500 m), Andes (average altitude, 4000 m), and East African Plateau (average altitude, 2400-3700 m).1,2 Low environmental O2 availability (hypoxia), dehydration, and low temperature are critical environmental challenges for vertebrates residing in these high-altitude regions.3 Hypoxia-induced erythropoiesis can occur in response to decreasing inspiratory O2 partial pressure, which requires more iron and may lead to progressive reductions in iron status and deficiency.4,5 Indeed, previous studies have reported low serum iron levels and bioavailability at high altitudes.6-10 Therefore, iron deficiency may increase health risks for residents at high altitude and can lead to the upregulation of transferrin, an endogenous plasma protein that binds to and transports iron.11
Inherited or acquired thromboembolic disorders are major causes of disability and death worldwide.12-15 High-altitude exposure is an important risk factor of thromboembolic disorders,16-19 such as venous thrombosis,20-23 pulmonary thromboembolism, mesenteric vein thrombosis, cerebral vein thrombosis, and deep vein thrombosis (DVT),16,24-26 due to blood hypercoagulation. Previous research has reported that a 1-year stay at high altitude is associated with a 30-times higher risk of thromboembolic events, including DVT and pulmonary embolism.19 Compared with low-altitude regions, long-term exposure to high altitudes is also associated with greater risk of stroke and associated hospitalization (1.05 in 1000 vs 13.7 in 1000 people, respectively).26 In addition, the incidence of venous thromboembolism is significantly higher in lowlanders exposed to high-altitude conditions,27 and according to a 5-year retrospective study of the US military academies, the incidence of thromboembolic events is 2 times higher at elevated altitudes (2210 m) than at sea level.28
Although many studies have investigated the causes of high altitude–induced thromboembolic disorders, reports are contradictory, and the underlying mechanisms remain poorly understood.16 Our group recently identified transferrin, an iron transport protein in plasma, as a prothrombotic protein that promotes blood coagulation.29,30 Our previous work shows that transferrin is sequestered by binding with fibrinogen at a molar ratio of 4:1 in normal conditions, whereas abnormally upregulated transferrin potentiates thrombin/factor XIIa (FXIIa) and inhibits antithrombin, thus inducing hypercoagulability.29 However, whether the upregulation of transferrin promotes hypercoagulability at high altitudes is unclear. In this study, we compared the concentration and activity of coagulation factors in plasma collected from both humans and mice exposed to different altitudes. Results showed that the activities of thrombin and FXIIa were significantly increased in the plasma of long-term human residents and short-stay mice at high altitude, although their concentrations did not change significantly. The increase in these activities coincided with the promotion of expression of the transferrin gene and an increase in the concentrations of transferrin in plasma. In mouse models, hypoxia- and low temperature–induced hypercoagulability and thrombosis aggravation were reversed by transferrin knockdown (RNR-Tf), transferrin antibody (Tf-AB) treatment, and peptide interference, which inhibited the potentiation of transferrin on thrombin and FXIIa. Thus, our study demonstrated that transferrin was upregulated by hypoxia and low temperature and acts as a key etiological factor in high altitude–induced thromboembolic disorders.
Materials and methods
Ethics statement and animals
All human samples were approved by the institutional review boards of the Kunming Institute of Zoology, Kunming, China; Second Affiliated Hospital of Guangxi Medical University, Nanning, China; First Affiliated Hospital of Kunming Medical University, Kunming, China; and People’s Hospital of Diqing Tibetan Autonomous Prefecture, Shangri-La, China (SMKX-20190115-09, 2019-KY [0118], 2022 Ethical Review L #13, and 2019 Ethical Review L #2, respectively) and were performed in accordance with the Declaration of Helsinki. Native, permanent residents who had lived locally for at least 5 years were included in this study. All specimens were collected between 2019 and 2020, with informed consent obtained from the participants before the study. All animal experiments were approved by the Animal Care and Use Committee at the Kunming Institute of Zoology (SMKX-TZ-2019.9.28-32-01) and conformed to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press, Eighth Edition, 2011). BALB/c mice (male, aged 7 weeks) were purchased from Vital River Experiment Animal Company (Beijing, China) and housed in a pathogen-free environment. Mice were kept in a sterile isolator and reared with autoclaved food and water at 24°C under a 12-hour light/dark cycle. Mice were anesthetized with pentobarbital sodium (60 mg/kg) via intraperitoneal injection. After recovery from anesthesia, the animals were given a standard diet and water ad libitum. Euthanasia was performed by cervical disarticulation while the mice were under a surgical plane of anesthesia. The animals were randomly assigned to operators by independent personnel not involved in data collection or analysis. The experimental group was blindly subjected to surgery, and all readout parameters were evaluated.
Collection of blood samples
Plasma samples at low (n = 111; males, 54; females, 57; age, 18-90 years), mid (n = 115; males, 66; females, 49; age, 16-87 years), and high (n = 128; males, 67; females, 61; age, 17-85 years) altitudes were collected from healthy volunteers at the Second Affiliated Hospital of Guangxi Medical University (∼79 m), First Affiliated Hospital of Kunming Medical University (∼1891 m), and People’s Hospital of Diqing Tibetan Autonomous Prefecture (∼3459 m), respectively. Mice (n = 90) were first kept at low altitude (∼79 m, Nanning, China) for 10 days.31 Thereafter, 30 mice were anesthetized with pentobarbital sodium (60 mg/kg) via an intraperitoneal injection for blood collection, with the remaining 60 mice moved to mid-altitude (∼1891 m, Kunming, China) and high-altitude (∼3459 m, Diqing, China) regions for 10 days, followed by blood collection. The plasma was obtained by centrifugation immediately after blood collection (using 3.8% sodium citrate as an anticoagulant), and then the plasma was aliquoted and stored at −80°C for further experiments, as described previously.29,30 O2 content and ambient temperature at different altitudes were determined by using an O2 detector (AS8801, SMART SENSOR, Dongguan, China) and a thermometer (TH702F, Anymetre, Guangzhou, China), respectively.
Concentrations of iron and proteins in plasma
The concentrations of iron and plasma proteins, including transferrin, prothrombin, fibrinogen, and FXII, were determined using a serum iron assay kit (ab83366, Abcam, Cambridge, MA) and enzyme-linked immunosorbent assay (ELISA) kits (SEC036Hu and CEC036Mu for human and mouse transferrin, respectively, CLOUD-CLONE, Wuhan, China; SEA710Hu and SEA710Mu for human and mouse prothrombin, respectively, CLOUD-CLONE, Wuhan, China; ab241383 and ab213478 for human and mouse fibrinogen, respectively, Abcam, Cambridge, MA; SEA677Hu and SEA677Mu for human and mouse FXII, respectively, CLOUD-CLONE, Wuhan, China), in accordance with the manufacturer’s instructions. Thrombin and FXIIa enzymatic activity assays were performed using the method described in supplemental Methods (available on the Blood website).
Mouse models induced by hypoxia or low temperature
BALB/c mice (male, aged 7 weeks) were randomly exposed to either hypoxia at 6% O2 in a hypoxic chamber (A-15274-P, Biospherix, Lacona, NY) for 3 days or low temperature at 0°C in a low-temperature incubator (ICP750, Memmert, Germany) for 10 days. The hypoxia- and low temperature–exposed mice were then further treated (through the caudal vein) with transferrin-depleting antibodies (once per day; 50 μg per injection), isotype control immunoglobulin G (IgG) (once per day, 50 μg per injection), RNR-Tf virus, blank (RNR) virus, hypoxia-inducible factor 1α (HIF-1α) inhibitor (LW6 [CAY10585]; once per day, 15 mg/kg injection; S8441, Selleck, Houston, TX), and the TH16 or FX18 peptide, which inhibits transferrin-thrombin/FXIIa interactions (once per day, 5 mg/kg).29,30 After 3 or 10 days of induction by hypoxia or low temperature, blood was collected (anticoagulant, 3.8% sodium citrate), and plasma was obtained by centrifugation for further assay. Bleeding time, carotid artery thrombosis model, DVT model, and stroke model were analyzed in normal environment after induction by hypoxia or low temperature.
FeCl3-induced carotid artery thrombosis
Mice were anesthetized by isoflurane inhalation with an anesthesia respirator (R540IP, RWD Life Science, Shenzhen, China), as described previously.29,30,32-34 The carotid artery was separated to induce thrombosis using 10% iron (III) chloride (FeCl3) soaked into a filter-paper disc (diameter = 2 mm). Blood flow was measured by laser speckle perfusion imaging (PeriCam PSI, Sweden) at 5 and 10 minutes after FeCl3 induction. The perfusion unit was recorded to quantify blood flow.
APTT and PT assays
To test the activated partial thromboplastin time (APTT), 50 μL of plasma was incubated with 50 μL of APTT reagent (F008-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for 3 minutes at 37°C; next, 50 μL of calcium chloride (25 mM) preheated at 37°C was added to test clotting time. Absorbance was monitored at 650 nm using a semiautomatic coagulation analyzer (Thrombo Screen 400c, Pacific Hemostasis, Huntersville, NC), as described previously.29,30 A prothrombin time (PT) assay kit (F007, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used following our previous research.29,30
Mouse stroke model
A transient middle cerebral artery occlusion (tMCAO) model was applied to induce focal cerebral ischemia according to previous research.30,35 A servo-controlled heating blanket was used to maintain a core body temperature close to 37°C throughout surgery. Briefly, mice were anesthetized with pentobarbital sodium (60 mg/kg) via intraperitoneal injection. After a midline skin incision in the neck, the proximal common carotid artery and external carotid artery were ligated, and a standardized silicon rubber-coated nylon monofilament (6023910PK10, Doccol, Sharon, MA) was inserted and advanced through the right internal carotid artery to occlude the origin of the right middle cerebral artery. After 30 minutes, the mice were reanesthetized, and the occluding filament was removed to allow reperfusion. To determine ischemic brain volume, the mice were sacrificed 24 hours after tMCAO induction, and their brains were quickly removed using a mouse brain slice matrix (Harvard Apparatus, Holliston, MA), cut into 2-mm-thick coronal slices, and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, St Louis, MO). Bederson and grip test scores were used to monitor neurological and motor function, respectively. Certain mice were excluded from end point analysis based on previously reported criteria.30
Statistical analysis
The normal distributions of all data were assessed using the Kolmogorov-Smirnov test, with values expressed as means ± standard deviation (SD). All statistical analyses were 2-tailed with 95% confidence intervals. Nonparametric data were compared using the Mann-Whitney U test. If only 2 groups were compared, unpaired t test was applied. GraphPad Prism 9 (GraphPad Software, San Diego, CA) and SPSS (SPSS Inc, Chicago, IL) were used for statistical analysis. P < .05 was considered statistically significant.
Results
Increased plasma thrombin and FXIIa activities are associated with altitude in both long-term human residents and short-stay mice
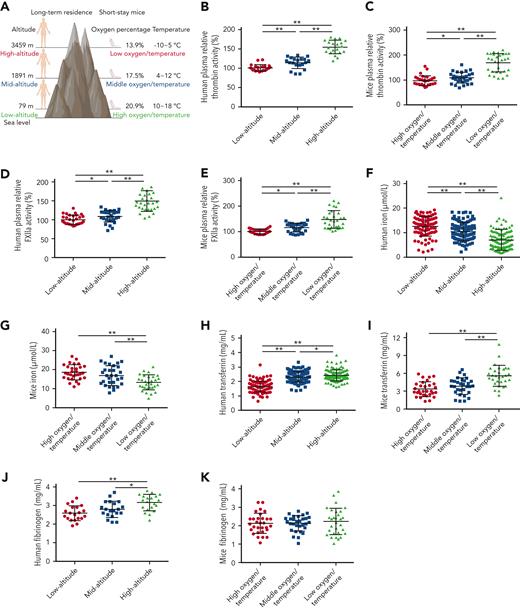
To investigate the mechanism responsible for altitude-associated hypercoagulability, enzymatic activities of coagulation factors, including FXIIa and thrombin, were determined in human and mouse plasma collected at different altitudes (Figure 1A). In comparison with low altitude, the average enzymatic activity of thrombin in plasma from long-term human residents at mid and high altitudes increased 0.13- and 0.54-fold and in short-stay mice increased 0.15- and 0.74-fold, respectively. Similarly, the enzymatic activity of FXIIa in human plasma at mid and high altitudes increased 0.14- and 0.5-fold and in mouse plasma increased 0.16- and 0.47-fold, respectively (Figure 1B-E). These findings indicate that increasing altitude is associated with enhanced plasma enzymatic activities of thrombin and FXIIa.
Enhanced thrombin and FXIIa activities, decreased iron levels, and elevated transferrin levels in plasma of long-term human residents and short-stay mice. (A) Graphical representation of plasma collection from long-term human residents and short-stay mice at different altitudes. (B-C) Relative enzymatic activity of thrombin in human (n = 20) (B) and mouse (n = 30) (C) plasma at different altitudes. (D-E) Relative enzymatic activity of FXIIa in human (n = 30) (D) and mouse (n = 30) (E) plasma at different altitudes. (F-G) Iron level in human (n = 111-128) (F) and mouse (n = 30) (G) plasma at different altitudes. (H-I) Transferrin concentration in human (n = 111-128) (H) and mouse (n = 30) (I) plasma at different altitudes. (J-K) Fibrinogen concentration in human (n = 20) (J) and mouse (n = 30) (K) plasma at different altitudes. Each experiment was independently repeated in triplicate. Data represent mean ± SD. Panels B, G, J, and K, ∗P < .05, ∗∗P < .01 by unpaired t test; panels C-F, H, and I, ∗P < .05, ∗∗P < .01 by Mann-Whitney U test.
Enhanced thrombin and FXIIa activities, decreased iron levels, and elevated transferrin levels in plasma of long-term human residents and short-stay mice. (A) Graphical representation of plasma collection from long-term human residents and short-stay mice at different altitudes. (B-C) Relative enzymatic activity of thrombin in human (n = 20) (B) and mouse (n = 30) (C) plasma at different altitudes. (D-E) Relative enzymatic activity of FXIIa in human (n = 30) (D) and mouse (n = 30) (E) plasma at different altitudes. (F-G) Iron level in human (n = 111-128) (F) and mouse (n = 30) (G) plasma at different altitudes. (H-I) Transferrin concentration in human (n = 111-128) (H) and mouse (n = 30) (I) plasma at different altitudes. (J-K) Fibrinogen concentration in human (n = 20) (J) and mouse (n = 30) (K) plasma at different altitudes. Each experiment was independently repeated in triplicate. Data represent mean ± SD. Panels B, G, J, and K, ∗P < .05, ∗∗P < .01 by unpaired t test; panels C-F, H, and I, ∗P < .05, ∗∗P < .01 by Mann-Whitney U test.
Iron and its transporter transferrin are decreased and increased, respectively, in plasma of human or mice plasma at mid and high altitudes
Decreased iron levels were observed in all mid- and high-altitude plasma samples (Figure 1F-G), suggesting reduced iron bioavailability at mid and high altitude, as reported in earlier studies.4,5 As a main iron transporter in plasma, transferrin has been found to potentiate the enzymatic activities of thrombin and FXIIa.29,30 Here, compared with the plasma transferrin concentration in healthy participants at low altitude (1.64 mg/mL [SD, 0.24]; n = 111; males, 54; females, 57; age, 18-90 years), the concentration at mid (n = 115; males, 66; females, 49; age, 16-87 years) and high (n = 128; males, 67; females, 61; age, 17-85 years) altitudes increased by 41.4% (2.32 mg/mL [SD, 0.31]) and 47.6% (2.42 mg/mL [SD, 0.50]), respectively. There was no correlation between age and transferrin level (supplemental Figure 1). Similarly, in comparison with the plasma concentration of transferrin in short-stay mice at low altitude (3.3 mg/mL; n = 30; SD, 1.24), the concentrations at mid and high altitudes increased by 9% and 78.8%, respectively, to 3.6 mg/mL (n = 30; SD, 1.33) and 5.8 mg/mL (n = 30; SD, 1.94), respectively. Elevated plasma transferrin levels at mid and high altitudes were further confirmed by western blot analysis (supplemental Figure 2A-B,E-F). The upregulation of transferrin appeared to be feedback on decreased iron availability.
Because transferrin (typically ∼40 μM) is primarily sequestered by binding with fibrinogen (normally ∼10 μM) at a molar ratio of 4:1,29 we tested the effects of altitude on the concentration of fibrinogen in plasma. The average plasma fibrinogen concentrations in long-term human residents at low, mid, and high altitudes were 2.6 mg/mL (SD, 0.40), 2.8 mg/mL (SD, 0.40), and 3.1 mg/mL (SD, 0.43), respectively. No significant changes were observed in the short-stay mice. The increase in transferrin (48%-79%) was much greater than that of fibrinogen (2%-20%) at high altitude. Other plasma proteins, including prothrombin and FXII, showed no significant change with the increase in altitude (supplemental Figure 2C-D,G-L).
Increased transferrin expression under both hypoxia and cold temperature at high altitudes
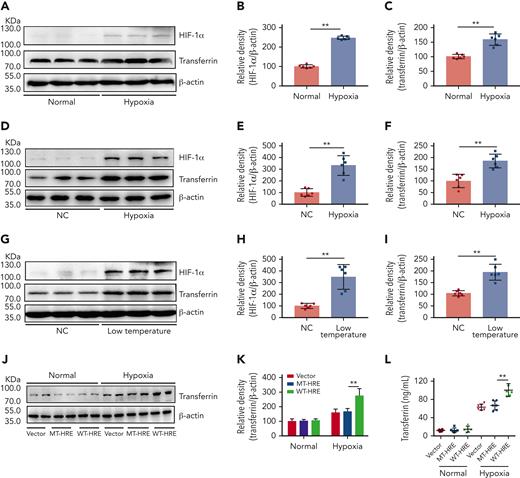
Exposure to the cold can induce tissue hypoxia and is considered one of the most damaging environmental factors at high altitude.36-38 Therefore, we investigated the effects of hypoxia on transferrin expression using a normal mouse liver cell line (BNL CL.2, Conservation Genetics CAS Kunming Cell Bank, China). Results showed that hypoxia upregulated the expression of both HIF-1α and transferrin in vitro (Figure 2A-C). To further elucidate the effects of high altitude on transferrin expression, BALB/c mice were subjected to hypoxia and low temperature stress. After either 3 days of hypoxia (6% O2) in a hypoxic chamber (Figure 2D-F) or 10 days of low-temperature exposure (0°C) in a low-temperature incubator (Figure 2G-I), substantial upregulation in transferrin and increase in HIF-1α were observed in the mouse liver in vivo, indicating that both hypoxia and low-temperature exposure can upregulate transferrin. Similar results were also reproduced at 8% O2 (supplemental Figure 3A-C). We previously reported that HIF-1α promotes transferrin gene expression by interacting with the enhancer region of the transferrin gene.30 In this study, mutation at the enhancer region hindered the effects of hypoxia on transferrin expression, as illustrated in Figure 2J-L. These results suggest that hypoxia and cold temperature at high altitude can increase the expression of transferrin.
Hypoxia- and low temperature–induced HIF-1α activation to promote transferrin expression both in vitro and in vivo. (A-C) After hypoxia treatment (1% O2, 5% CO2, and 94% N2), HIF-1α and transferrin proteins in BNL CL.2 cells were analyzed by western blot analysis (A), and corresponding quantifications are shown in panels B and C. β-actin was used as loading control in panel A (n = 6). (D-F) Western blot detection of HIF-1α and transferrin levels in livers of normal and hypoxia-induced mice and corresponding quantifications (n = 6). (G-I) Western blot analysis of HIF-1α and transferrin levels in liver of normal and low temperature–induced mice and corresponding quantifications (n = 6). β-actin was used as loading control in panels D and G. (J-L) Transferrin levels in supernatant of HepG2 cells transfected by transferrin expression plasmid of wild-type hypoxia response elements (HRE; WT-HRE) or mutated HRE (MT-HRE) were analyzed by western blot analysis (J,K) and ELISA (L) after hypoxia treatment (n = 6). β-actin was used as loading control in panel J. Each experiment was independently repeated in triplicate. Data represent mean ± SD. Panels B, C, E, F, H, I, K, and L, ∗∗P < .01 by unpaired t test. Western blots were from different membranes, and representative blots are shown in panels A, D, G, and J. NC, normal control.
Hypoxia- and low temperature–induced HIF-1α activation to promote transferrin expression both in vitro and in vivo. (A-C) After hypoxia treatment (1% O2, 5% CO2, and 94% N2), HIF-1α and transferrin proteins in BNL CL.2 cells were analyzed by western blot analysis (A), and corresponding quantifications are shown in panels B and C. β-actin was used as loading control in panel A (n = 6). (D-F) Western blot detection of HIF-1α and transferrin levels in livers of normal and hypoxia-induced mice and corresponding quantifications (n = 6). (G-I) Western blot analysis of HIF-1α and transferrin levels in liver of normal and low temperature–induced mice and corresponding quantifications (n = 6). β-actin was used as loading control in panels D and G. (J-L) Transferrin levels in supernatant of HepG2 cells transfected by transferrin expression plasmid of wild-type hypoxia response elements (HRE; WT-HRE) or mutated HRE (MT-HRE) were analyzed by western blot analysis (J,K) and ELISA (L) after hypoxia treatment (n = 6). β-actin was used as loading control in panel J. Each experiment was independently repeated in triplicate. Data represent mean ± SD. Panels B, C, E, F, H, I, K, and L, ∗∗P < .01 by unpaired t test. Western blots were from different membranes, and representative blots are shown in panels A, D, G, and J. NC, normal control.
Increased transferrin induced by hypoxia and low temperature potentiates thrombin/FXIIa in vivo
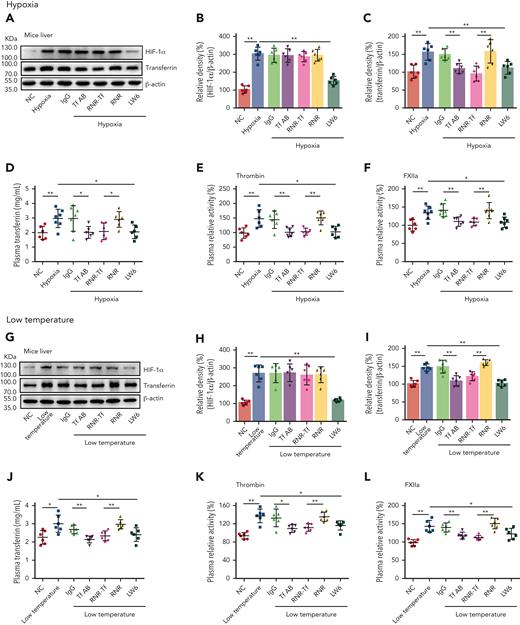
As illustrated in Figure 3A-D,G-J, the levels of both transferrin and HIF-1α in the liver of BALB/c mice were upregulated when exposed to hypoxia and low temperature, as revealed by western blot analysis. The ELISA results also demonstrated that plasma levels of transferrin were upregulated under hypoxic and low-temperature conditions. Tf-AB, RNR-Tf, and HIF-1α inhibitor LW6 reversed both hypoxia- and low temperature–induced transferrin upregulation. Interestingly, HIF-1α upregulation was also reversed by the aforementioned treatments, suggesting potential reciprocal interactions between HIF-1α and transferrin expression. As expected, the isotype IgG and blank virus controls (with empty knockdown [RNR] vector) showed no effects. As illustrated in Figure 3E-F,K-L, with the increase in transferrin and HIF-1α, the enzymatic activities of thrombin and FXIIa in the plasma of hypoxia-induced mice also significantly increased. However, the increase in enzymatic activities was reversed by Tf-AB, RNR-Tf, and LW6 treatment, with the IgG and blank virus controls showing no significant effects. As illustrated in supplemental Figure 4, there was no significant difference in iron level in the different groups of mice described earlier.
Hypoxia- and low temperature–induced transferrin upregulation to potentiate enzymatic activities of thrombin and FXIIa. (A-D,G-J) Effects of anti–Tf-AB, IgG control, RNR-Tf virus, blank (RNR) virus, and HIF inhibitor LW6, on HIF-1α and transferrin expression in liver or transferrin expression in plasma of mice following hypoxia and low-temperature treatment was determined by western blotting (A-C,G-I) or ELISA (D,J) (n = 6-7). (E-F,K-L) Relative activities of thrombin (E and K) and FXIIa (F,L) in plasma were examined (n = 6-7). β-actin was used as loading control in panels A and G. Animal experiments were repeated 3 times, independently. Data represent mean ± SD. Each point represents 1 mouse. Panels B-F and H-L, ∗P < .05, ∗∗P < .01 by unpaired t test. Western blots were from different membranes, and representative blots are shown in panels A and G.
Hypoxia- and low temperature–induced transferrin upregulation to potentiate enzymatic activities of thrombin and FXIIa. (A-D,G-J) Effects of anti–Tf-AB, IgG control, RNR-Tf virus, blank (RNR) virus, and HIF inhibitor LW6, on HIF-1α and transferrin expression in liver or transferrin expression in plasma of mice following hypoxia and low-temperature treatment was determined by western blotting (A-C,G-I) or ELISA (D,J) (n = 6-7). (E-F,K-L) Relative activities of thrombin (E and K) and FXIIa (F,L) in plasma were examined (n = 6-7). β-actin was used as loading control in panels A and G. Animal experiments were repeated 3 times, independently. Data represent mean ± SD. Each point represents 1 mouse. Panels B-F and H-L, ∗P < .05, ∗∗P < .01 by unpaired t test. Western blots were from different membranes, and representative blots are shown in panels A and G.
Reversal of hypercoagulability induced by hypoxia and low temperature by RNR-Tf and functional interference
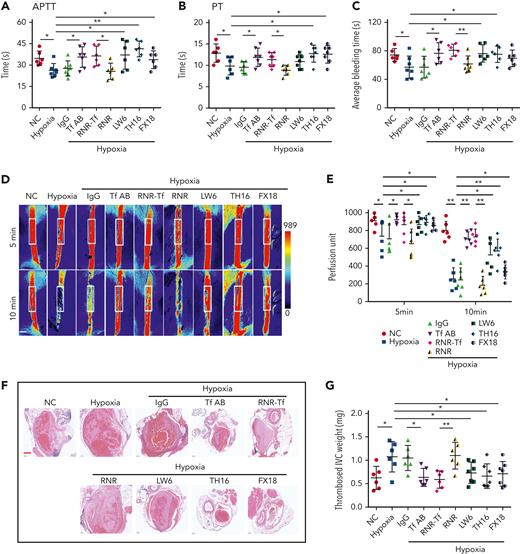
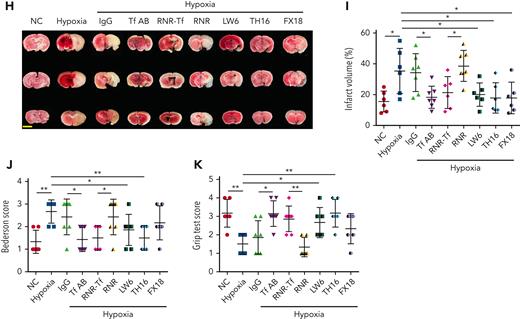
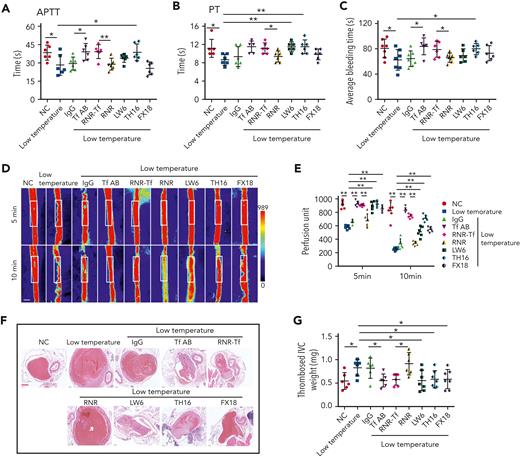
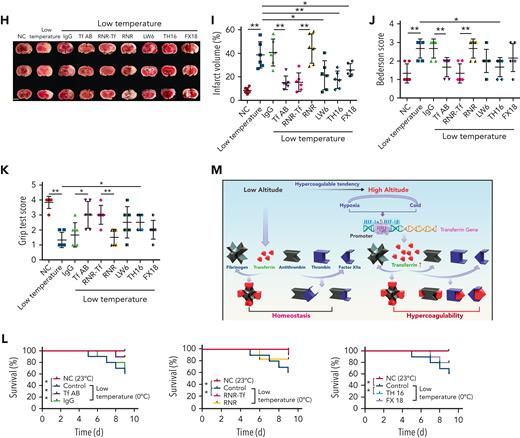
Hypoxia and low temperature increased the enzymatic activities of thrombin and FXIIa in plasma, suggesting that they may induce hypercoagulability. This was confirmed by the reductions in APTT, PT, and bleeding time in mice subjected to hypoxia (Figure 4A-C). Importantly, Tf-AB, RNR-Tf, LW6, and the peptides TH16 and FX18, which interfere with transferrin-thrombin/FXIIa interactions,29,30 reversed the reductions in APTT, PT, and bleeding time, whereas the IgG and blank virus controls showed no effects (Figure 4A-C). Consistent with the reductions in APTT, PT, and bleeding time, decreased blood flow and aggravated thrombus formation were also observed in the carotid arteries and deep vein of hypoxia-induced mice. Notably, Tf-AB, RNR-Tf, LW6, TH16, and FX18 reversed the reduction in blood flow and aggravation of thrombus in hypoxia-treated mice (Figure 4D-G). Moreover, the effects of RNR-Tf and functional interference on thrombosis and ischemic stroke (IS) aggravated by hypoxia were investigated using the tMCAO mouse model. As illustrated in Figure 4H, hypoxia induced a significant increase in infarct volume (Figure 4H-I) and severe functional outcomes, as represented by an increased Bederson score and decreased grip scores (Figure 4J-K). Transferrin functional interference by Tf-AB, RNR-Tf, LW6, TH16, and FX18 inhibited thrombosis and IS aggravation induced by hypoxia in the tMCAO mouse model, whereas the IgG and blank virus controls had no effects (Figure 4H-K). Similarly, thrombosis and IS aggravation induced by low temperature were reversed by RNR-Tf and functional interference (Figure 5A-K). Importantly, on day 9 after low-temperature exposure, mice that received RNR-Tf or functional interference showed higher survival trends than the controls (60%), with survival rates of 90%, 90%, 80%, and 80% in the Tf-AB–, RNR-Tf–, Th16-, and FX18-treated groups, respectively (Figure 5L). Notably, combined hypoxia and low-temperature condition induced hypercoagulability and thrombosis formation, which was more severe than hypoxia induction alone. Tf-AB reversed the prothrombotic tendency in this combined condition (supplemental Figure 5). As illustrated in supplemental Figure 6A, although transferrin was elevated by combined hypoxia with low-temperature induction, thrombin-specific inhibitor dabigatran or FXIIa antibody intervention alleviated hypercoagulability and thrombus formation (supplemental Figure 6B-E). All these data suggest that RNR-Tf and functional interference provide considerable protection against the insults caused by hypoxia or low temperature.
Hypoxia-induced hypercoagulability and thrombosis aggravation, which were reversed by transferrin knockdown and functional interference. (A-C) Effects of anti–Tf-AB, IgG control, RNR-Tf virus, blank (RNR) virus, HIF inhibitor LW6, or peptides TH16 and FX18 on APTT (A), PT (B), and bleeding time (C) in hypoxia-treated mice (n = 6-8). (D-E) Representative images of carotid artery blood flow (left) in FeCl3-treated mice by laser speckle perfusion imaging (D), with region of interest (rectangle in white) placed in carotid artery to quantify blood flow change (E) (n = 6). Color bar at bottom indicates perfusion unit scale (0-989); scale bar represents 1 mm. Mice were subject to inferior vena cava (IVC) stenosis for 24 hours to evaluate venous thrombogenesis. (F-G) The pathological changes were observed through hematoxylin and eosin staining (F) and calculating thrombus weight (G) (n = 6-7); scale bar represents 200 μm. (H-I) Representative images of TTC-stained coronal brain sections (H) and quantitative analysis of stained area (I) on day 1 after tMCAO. Ischemic infarctions appear white, and brain infarct volumes were measured by planimetry (percentage of whole volume). (J-K) Bederson (J) and grip test (K) scores were also measured (n = 6-7); scale bar represents 0.5 cm. Animal experiments were repeated 3 times, independently. Data represent mean ± SD. Each point represents 1 mouse. Panels A-C, E, G, and I-K, ∗P < .05, ∗∗P < .01 by unpaired t test to compare; for example, Tf-AB and IgG groups or RNR-Tf and RNR groups.
Hypoxia-induced hypercoagulability and thrombosis aggravation, which were reversed by transferrin knockdown and functional interference. (A-C) Effects of anti–Tf-AB, IgG control, RNR-Tf virus, blank (RNR) virus, HIF inhibitor LW6, or peptides TH16 and FX18 on APTT (A), PT (B), and bleeding time (C) in hypoxia-treated mice (n = 6-8). (D-E) Representative images of carotid artery blood flow (left) in FeCl3-treated mice by laser speckle perfusion imaging (D), with region of interest (rectangle in white) placed in carotid artery to quantify blood flow change (E) (n = 6). Color bar at bottom indicates perfusion unit scale (0-989); scale bar represents 1 mm. Mice were subject to inferior vena cava (IVC) stenosis for 24 hours to evaluate venous thrombogenesis. (F-G) The pathological changes were observed through hematoxylin and eosin staining (F) and calculating thrombus weight (G) (n = 6-7); scale bar represents 200 μm. (H-I) Representative images of TTC-stained coronal brain sections (H) and quantitative analysis of stained area (I) on day 1 after tMCAO. Ischemic infarctions appear white, and brain infarct volumes were measured by planimetry (percentage of whole volume). (J-K) Bederson (J) and grip test (K) scores were also measured (n = 6-7); scale bar represents 0.5 cm. Animal experiments were repeated 3 times, independently. Data represent mean ± SD. Each point represents 1 mouse. Panels A-C, E, G, and I-K, ∗P < .05, ∗∗P < .01 by unpaired t test to compare; for example, Tf-AB and IgG groups or RNR-Tf and RNR groups.
Low temperature–induced hypercoagulability and thrombosis aggravation, which were reversed by transferrin knockdown and functional interference. (A-C) APTT (A), PT (B), and bleeding time (C) in mice are shown (n = 6-7). (D-E) Representative images of carotid artery blood flow in FeCl3-treated mice by laser speckle perfusion imaging (D), with region of interest (rectangle in white) placed in carotid artery to quantify blood flow change (E) (n = 6). Color bar at bottom indicates perfusion unit scale (0-989); scale bar represents 1 mm. Mice were subject to IVC stenosis for 24 hours to evaluate venous thrombogenesis. (F-G) The pathological changes were observed through hematoxylin and eosin staining (F) and calculating thrombus weight (G) (n = 6-7); scale bar represents 200 μm. (H-I) Representative images of TTC-stained coronal brain sections (H) and quantitative analysis of stained area (I) on day 1 after tMCAO. Ischemic infarctions appear white, and brain infarct volumes were measured by planimetry (percentage of whole volume). (J-K) Bederson (J) and grip-test (K) scores were also measured (n = 6). (L) Survival rate of mice in anti-Tf-AB–, RNR-Tf–, RNR virus–, and TH16- and FX18-peptide–treated groups under low temperature (n = 20). (M) Graphical representation of iron and O2 replenishment at high altitude, contributing to thromboembolic disorders. Detrimental environmental factors (hypoxia and low temperature) and high altitude–induced iron deficiency increased HIF-1α levels, which upregulated transferrin to increase iron transport to compensate for erythropoiesis and O2 supply. Simultaneously, abnormally upregulated transferrin caused hypercoagulability by interacting with multiple plasma proteins to potentiate thrombin and FXIIa and inhibit antithrombin. Animal experiments were repeated 3 times, independently. Data represent mean ± SD. Each point represents 1 mouse. Panels A-C, E, G, and I-K, ∗P < .05, ∗∗P < .01 by unpaired t test to compare; for example, Tf-AB and IgG groups or RNR-Tf and RNR groups. Panel L, ∗P < .05 by log-rank test to compare; for example, Tf-AB and IgG groups or RNR-Tf and RNR groups.
Low temperature–induced hypercoagulability and thrombosis aggravation, which were reversed by transferrin knockdown and functional interference. (A-C) APTT (A), PT (B), and bleeding time (C) in mice are shown (n = 6-7). (D-E) Representative images of carotid artery blood flow in FeCl3-treated mice by laser speckle perfusion imaging (D), with region of interest (rectangle in white) placed in carotid artery to quantify blood flow change (E) (n = 6). Color bar at bottom indicates perfusion unit scale (0-989); scale bar represents 1 mm. Mice were subject to IVC stenosis for 24 hours to evaluate venous thrombogenesis. (F-G) The pathological changes were observed through hematoxylin and eosin staining (F) and calculating thrombus weight (G) (n = 6-7); scale bar represents 200 μm. (H-I) Representative images of TTC-stained coronal brain sections (H) and quantitative analysis of stained area (I) on day 1 after tMCAO. Ischemic infarctions appear white, and brain infarct volumes were measured by planimetry (percentage of whole volume). (J-K) Bederson (J) and grip-test (K) scores were also measured (n = 6). (L) Survival rate of mice in anti-Tf-AB–, RNR-Tf–, RNR virus–, and TH16- and FX18-peptide–treated groups under low temperature (n = 20). (M) Graphical representation of iron and O2 replenishment at high altitude, contributing to thromboembolic disorders. Detrimental environmental factors (hypoxia and low temperature) and high altitude–induced iron deficiency increased HIF-1α levels, which upregulated transferrin to increase iron transport to compensate for erythropoiesis and O2 supply. Simultaneously, abnormally upregulated transferrin caused hypercoagulability by interacting with multiple plasma proteins to potentiate thrombin and FXIIa and inhibit antithrombin. Animal experiments were repeated 3 times, independently. Data represent mean ± SD. Each point represents 1 mouse. Panels A-C, E, G, and I-K, ∗P < .05, ∗∗P < .01 by unpaired t test to compare; for example, Tf-AB and IgG groups or RNR-Tf and RNR groups. Panel L, ∗P < .05 by log-rank test to compare; for example, Tf-AB and IgG groups or RNR-Tf and RNR groups.
Discussion
O2 and iron are essential for most organisms. In response to decreased inspiratory O2 partial pressure at high altitudes, erythropoiesis occurs to increase O2 availability and enhance iron consumption, which can lead to iron deficiency.6-10 In this study, the physiological mechanisms underlying the replenishment of iron and O2 and the association with high altitude–induced thromboembolic disorders were investigated. We report that the plasma concentration of transferrin, a known iron transporter, was significantly elevated in human plasma at high altitude (Figure 1H). Both hypoxia and low-temperature stimulation in vitro and in vivo increased HIF-1α levels and promoted the expression of the transferrin gene, which contains HIF-1α binding sites in its enhancer region. Consistent with the upregulation of transferrin, thrombotic complications were induced in mice stimulated by hypoxia or low temperature. Transferrin depletion via antibody treatment, RNR-Tf, and interference of transferrin potentiation of thrombin and FXIIa enzymatic activities inhibited hypoxia- and low temperature–augmented thrombosis in FeCl3, DVT, and tMCAO mouse models. Our results revealed that transferrin upregulation, which appears to be a physiological compensation mechanism to replenish iron and O2 at high altitude, plays a key role in high altitude–induced thromboembolic disorders.
As the principal iron transporter, the concentration of transferrin in plasma of healthy humans is usually ∼40 μM.29 We recently reported that transferrin interacts with fibrinogen, thrombin, and FXIIa with different affinities to maintain coagulation balance.29,30 Transferrin is primarily sequestered by binding to fibrinogen (normal plasma concentration, ∼10 μM) at a molar ratio of 4:1.29,30 In this study, although plasma fibrinogen increased at high altitude, its increase (2%-20%) was significantly less than that of transferrin (48%-79%) (Figure 1H-K). Transferrin potentiated the enzymatic activities of thrombin/FXIIa, thereby inducing hypercoagulability and increasing susceptibility to thromboembolic disorders. These results further confirmed the upregulation of transferrin with altitude and its important role in regulating coagulation as a moonlighting protein in the mediation of multiple protein-protein interactions.
Blood coagulation and platelet activation are complementary and mutually dependent processes in vivo. Thrombin is a potent platelet agonist, which acts on proteinase-activated receptors in platelets. The generation or activation of thrombin by the coagulation cascade can activate platelets, which, in turn, secrete coagulation factors and provide negatively charged surfaces to support cell-based thrombin generation and coagulation.39-41 Although coagulation may activate platelets, previous research has reported that increased thrombin generation caused by high altitude is balanced by reduced platelet activity.42,43 Some authors have reported a decrease in platelet number when exposed to high altitude.44,45 Although a previous report showed that platelet activity was decreased by hypothermia,46 more studies reported that platelet function and number were elevated by low temperature.47-49 Although beyond the scope of this study, systematic research on the relationship between platelets, including these platelets in aged populations,50-52 and thromboembolic disorders at high altitude would be worthwhile. Furthermore, studying the dynamic changes in transferrin and coagulation activities in travelers who have moved from sea level to high-altitude regions (or vice versa) would be interesting.
Hypoxia and low temperature are detrimental environmental factors at high altitude. Previous studies have demonstrated that cold temperature exposure can induce tissue hypoxia, resulting in the upregulation of HIF expression.36-38 Under hypoxic conditions, HIF is rapidly stabilized in cells, thus allowing it to regulate the expression of hundreds of genes that promote adaptive responses to hypoxia.53,54 Hypoxia is a state in which the body or tissue is deprived of an adequate O2 supply. O2 metabolism and iron homeostasis are closely linked, with iron facilitating the O2-carrying capacity of blood.55 Exposure to low atmospheric O2 at high altitude can lead to erythropoietin synthesis for red blood cell production, which in turn increases the demand for iron to synthesize hemoglobin.56 HIF activation under low cellular iron concentrations, hypoxia, and low temperature regulates the transcription of genes involved in iron utilization, such as transferrin54 and its receptor TFR1.57,58 Our recent work indicated that transferrin expression is promoted by HIF.29,30 In this study, as illustrated in Figure 2D,G, transferrin was significantly upregulated in response to hypoxia or low-temperature stimulation. Previous reports showed a significant increase in plasma transferrin concentrations in the blood of winter swimmers.59 On the one hand, increased transferrin carries iron to various tissues and cells for red blood cell production to compensate for the reduced availability of O2 at high altitudes. On the other hand, elevated transferrin in plasma promotes hypercoagulability by acting as a prothrombotic factor, thereby suggesting a physiological trade-off strategy.
Increased thromboembolic events occur at high altitude, which is of importance from both a scientific and clinical standpoint. Previous studies reported that increase of HIF-1α was associated with reduced protein S expression and HIF-1α–dependent increases in tissue factor–triggered thrombus formation by hypoxia.60-62 Here, transferrin was identified as a key etiological factor involved in these events. As illustrated in Figures 4 and 5, hypoxia and low temperature promoted hypercoagulability, as characterized by decreased blood flow, aggravated thrombus formation, ischemia, APTT, and PT, as well as increased plasma transferrin levels. These characterizations were reversed by functionally blocking the interactions of transferrin with other coagulation factors. Furthermore, functional blockages of transferrin or RNR-Tf significantly decreased mice mortality induced by low temperature. Taken collectively, our results indicate that transferrin is upregulated by hypoxia and low temperature and acts as a key mediator to promote hypercoagulability at high altitude. Harmful environmental factors under high-altitude conditions upregulate transferrin to carry more iron to compensate for erythropoiesis and O2 supply. Simultaneously, the abnormally upregulated transferrin causes hypercoagulability by potentiating thrombin and FXIIa and inhibiting antithrombin. Notably, anti–Tf-AB, RNR-Tf, and TH16 peptide treatment almost completely inhibited the hypercoagulability induced by low temperature and hypoxia. These treatments showed considerable protective effects and provide a potential therapeutic target and promising strategy for the treatment of high altitude–induced thromboembolic disorders.
Acknowledgments
The authors thank Peng Shi and Yongbin Chen for providing the hypoxic chamber.
This work was supported by grants from the National Science Foundation of China (31930015, 81770464, and 32100907); Ministry of Science and Technology of China (2018YFA0801403); the Chinese Academy of Sciences (XDB31000000, KFJ-STS-SCYD-304); the K. C. Wong Education Foundation, Science and Technology Department of Yunnan Province (202202AA100002, 202003AD150008, 2019ZF003, 2019FA006, and 2019FI014), and Kunming Science and Technology Bureau (2023SCP001) as well as Canadian Institutes of Health Research Foundation (389035). C.S. is a recipient of a postdoctoral Mitacs award, University of Toronto. D.T.M. is a recipient of a graduate scholarship, Department of Physiology, University of Toronto, and a Queen Elizabeth II graduate scholarship. E.G.C. is a recipient of a St. Michael’s Hospital Research Training Centre scholarship and a Queen Elizabeth II graduate scholarship.
Authorship
Contribution: M.L., X.T., Z.L., C.S., R.C., M.F., G.W., Z.Z., P.M.K., and J.M. performed the experiments, including enzyme activity tests, animal experiments, and data analysis; Ya Li, S.T., L.X., Yaxiong Li, and Y.W. collected specimens from healthy participants at low, mid, and high altitudes; R.L., X.T., M.L., Q.L., D.T.M., and E.G.C. analyzed data and prepared the manuscript; R.L., H.N., and X.T. conceived and supervised the project; and all authors contributed to the discussions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ren Lai, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, Yunnan, China; e-mail: rlai@mail.kiz.ac.cn; Heyu Ni, Department of Laboratory Medicine and Pathobiology, Department of Medicine and Department of Physiology, University of Toronto and Canadian Blood Services Centre for Innovation, Hematology, Cancer and Immunological Diseases, St. Michael’s Hospital, LKSKI-Keenan Research Centre, Room 421, 209 Victoria St, Toronto, ON M5B 1W8, Canada; e-mail: heyu.ni@unityhealth.to; and Xiaopeng Tang, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming 650223, Yunnan, China; e-mail: tangxiaopeng@mail.kiz.ac.cn.
References
Author notes
∗M.L., X.T., and Z.L. contributed equally to this study.
Data are available on request from the corresponding authors, Ren Lai (rlai@mail.kiz.ac.cn), Heyu Ni (heyu.ni@unityhealth.to), and Xiaopeng Tang (tangxiaopeng@mail.kiz.ac.cn).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

Comments
Response
We have read the paper by Dr. Tyagi (Blood. 2014 Feb 20;123(8):1250-60). It is a very nice work, in which Dr. Tyagi and colleagues demonstrated a decreased bleeding propensity and increased platelet reactivity in rats exposed to acute simulated hypoxia. Using proteomic analysis, they found 27 differentially expressed proteins in hypoxic platelets including calpain small subunit 1 (CAPNS1) and several coagulation factors, such as fibrinogen and tissue factor precursor. They highlighted that calpains play a vital role in hypoxia-induced prothrombotic phenotype, and inhibition of calpain activity can reverse hypoxia-induced platelet hyperreactivity.
In our study, we found that the activities of thrombin and factor XIIa (FXIIa), along with the concentrations of transferrin, were significantly increased in the plasma of humans and mice at high altitudes. Furthermore, both hypoxia and low temperature enhanced hypoxia-inducible factor 1α (HIF-1α) levels, in turn promoting transferrin expression, and consequently inducing hypercoagulability by potentiating thrombin and FXIIa generation. Importantly, thromboembolic disorders and pathological insults in mouse models induced by both hypoxia and low temperature were ameliorated by transferrin interference, including transferrin antibody treatment, transferrin downregulation, and the administration of our newly developed peptides that inhibit the potentiation of transferrin on thrombin and FXIIa. Thus, low temperature and hypoxia upregulated transferrin expression promoted hypercoagulability. Our work indicates that hypoxic conditions in mice and/or humans living at high elevations led to the increased expression of transferrin, which can potentiate the function of thrombin and factor XIIa, thus enhancing thrombotic risk.
Obviously, the study by Dr. Tyagi et al., focuses on platelet proteins, while our work focuses on coagulation factors, transferrin regulation upon hypoxic and low temperature conditions via HIF-1α, and coagulation factor-transferrin interaction(s). Thus, regretfully, we did not cite the paper of Tyagi et al, which is also likely the reason for our reviewers. We look forward to citing our blood papers from both groups in our future work as both platelets and coagulation factors contribute to the thrombotic events at high altitudes.
Important previous related report missing
Thanks