Key Points
We established proof of concept for activating pyruvate kinase (PK) in sickle cell disease (SCD) as a viable therapeutic approach.
Mitapivat, a PK activator, improved hematologic parameters, increased oxygen affinity, and reduced sickling in patients with SCD anemia.
Abstract
Polymerization of deoxygenated hemoglobin S underlies the pathophysiology of sickle cell disease (SCD). In activating red blood cell pyruvate kinase and glycolysis, mitapivat (AG-348) increases adenosine triphosphate (ATP) levels and decreases the 2,3-diphosphoglycerate (2,3-DPG) concentration, an upstream precursor in glycolysis. Both changes have therapeutic potential for patients with SCD. Here, we evaluated the safety and tolerability of multiple ascending doses of mitapivat in adults with SCD with no recent blood transfusions or changes in hydroxyurea or l-glutamine therapy. Seventeen subjects were enrolled; 1 subject was withdrawn shortly after starting the study. Sixteen subjects completed 3 ascending dose levels of mitapivat (5, 20, and 50 mg, twice daily [BID]) for 2 weeks each; following a protocol amendment, the dose was escalated to 100 mg BID in 9 subjects. Mitapivat was well tolerated at all dose levels, with the most common treatment-emergent adverse events (AEs) being insomnia, headache, and hypertension. Six serious AEs (SAEs) included 4 vaso-occlusive crises (VOCs), non–VOC-related shoulder pain, and a preexisting pulmonary embolism. Two VOCs occurred during drug taper and were possibly drug related; no other SAEs were drug related. Mean hemoglobin increase at the 50 mg BID dose level was 1.2 g/dL, with 9 of 16 (56.3%) patients achieving a hemoglobin response of a ≥1 g/dL increase compared with baseline. Mean reductions in hemolytic markers and dose-dependent decreases in 2,3-DPG and increases in ATP were also observed. This study provides proof of concept that mitapivat has disease-modifying potential in patients with SCD. This trial was registered at www.clinicaltrials.gov as #NCT04000165.
Introduction
Sickle cell disease (SCD) is a debilitating disorder characterized by recurrent episodes of vaso-occlusive pain, hemolytic anemia, and progressive organ damage; affected individuals have a significantly shortened life expectancy.3,4 SCD is caused by the presence of hemoglobin S (HbS, βGlu6Val), the consequence of a single base mutation in the HBB gene (c.20A>T; rs334). Upon deoxygenation, HbS polymerizes into rigid fibers causing distortion (sickling) and damage to the red blood cells (RBCs), thereby promoting hemolysis and microvascular occlusion, leading to ischemia/reperfusion injuries that underlie the progressive organ damage in SCD.5-9 The condition, which primarily affects people of African descent, afflicts approximately 100 000 individuals in the United States and millions of individuals worldwide.10,11 Despite the global health burden, management strategies for SCD have evolved very slowly and historically have been neglected. Treatment options for SCD may be broadly classified into treatments that address the basic sickling process and treatments that target the downstream sequelae of sickling. Curative therapies including stem cell transplants from allogeneic donors and genetic therapies, currently under clinical development,12-14 are available to only a very small number of patients, limited either by donor availability or advanced medical facilities even in well-resourced countries with good health care provision. Meanwhile, safe and effective oral drug therapies are desperately needed for the vast majority of patients with SCD.15 To date, hydroxyurea is the only agent approved by the US Food and Drug Administration that directly affects sickling and decreases the frequency of pain episodes, which is of most concern to patients.16-18
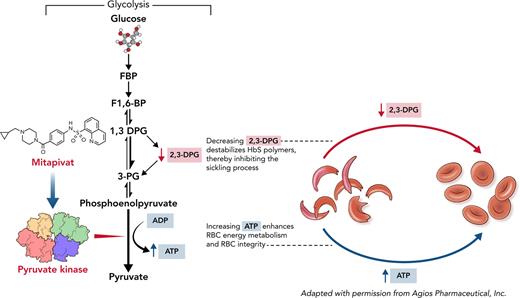
Several approaches apart from fetal Hb (HbF) induction can be considered for targeting the polymerization of HbS.19,20 HbS polymerizes only when at least partially deoxygenated. A key factor that influences deoxygenation is the RBC concentration of 2,3-diphosphoglycerate (2,3-DPG), an intermediate in glycolysis.21,22 2,3-DPG decreases oxygen affinity by preferentially binding to the low-affinity T conformation of HbS, stabilizing the HbS fiber.23,24 In addition, increased 2,3-DPG concentration decreases intra-erythrocyte pH, which promotes HbS polymerization.23-25 Furthermore, integrity of the RBC depends on adenosine triphosphate (ATP), and glycolysis is the main source of ATP production in anucleate RBCs.26 Therefore, increasing red cell pyruvate kinase (PKR) activity, which leads to an increase in ATP production and simultaneous decrease in RBC 2,3-DPG concentration, presents a new and attractive approach to reduce sickling and hemolysis by inhibiting HbS polymerization and maintaining RBC integrity.
Mitapivat (AG-348) is an orally bioavailable, small-molecule allosteric activator of PKR that has been shown to activate both wild-type and mutant variants of the enzyme.27 In healthy individuals, multiple-dose treatment with mitapivat had an acceptable safety profile and exhibited dose-dependent decreases in 2,3-DPG and increases in ATP levels, consistent with activation of the glycolytic pathway.27,28 Investigators also reported an acceptable safety profile with evidence of sustained hemoglobin increases from baseline in clinical studies involving patients with PKR deficiency.29 Phase 2/3 clinical trials for PKR deficiency and thalassemia are completed or ongoing (#NCT03559699, #NCT03548220, #NCT04770779, #NCT04770753).
In this phase 1, investigator-sponsored study, the safety and tolerability of multiple ascending doses of mitapivat have been evaluated for the first time in adults with SCD-HbSS.
Patients and methods
Details on study design, eligibility criteria, and analytical methodology are provided in supplemental Materials, available on the Blood website.
Study design
This was a single-arm, open-label study conducted at the National Institutes of Health (NIH) in Bethesda, MD. After a screening period, patients received either 3 or 4 ascending dose levels of mitapivat (5, 20, 50, or 100 mg twice daily [BID] by mouth) for a 2-week duration each, followed by a drug taper period of 9 to 15 days (supplemental Figure 1). The initial subjects escalated to a maximum dose of 50 mg BID, and subsequent subjects escalated up to a 100-mg BID dose following a protocol amendment. Early in the study, a vaso-occlusive crisis (VOC) occurred during drug taper, after which the protocol was consequently amended to increase the length of taper from 9 to 12 days for the 50-mg BID dose and 15 days for the 100-mg BID dose.
Patients
Subjects aged ≥ 18 years with a confirmed diagnosis of SCD (HbSS), adequate organ function, baseline Hb ≥ 7 g/dL, and no transfusions or erythropoietin therapy in the prior 3 months were eligible. Concomitant stable hydroxyurea (HU) and/or l-glutamine therapy was permitted on the study. Individuals of childbearing potential were required to use 2 forms of contraception during the study. Patients were permitted to receive blood transfusions and other clinically indicated treatments as needed on the study at the discretion of the principal investigator. Patients who received crizanlizumab or voxelotor in the 90 days before consent were excluded (see supplemental Appendix for full list of inclusion and exclusion criteria). Patients who required discontinuation of treatment before reaching the 50-mg dose level, self-discontinued treatment, or were lost to follow-up were withdrawn from the study.
Trial oversight
The study was designed by the investigator, sponsored by the National Heart, Lung, and Blood Institute (NHLBI), and the study drug was provided by Agios Pharmaceuticals. The trial protocol was approved by the NIH institutional review board. Written informed consent was obtained from all patients before enrollment. The data were reviewed on an ongoing basis by the NIH data and safety monitoring board. Trial data were collected and analyzed by authors employed by the NHLBI, and all authors had access to the primary trial data. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
End points and assessments
The primary objective of the study was to assess the safety and tolerability of mitapivat in all patients who received at least 1 dose of the drug. We evaluated frequency and severity of adverse events (AEs) that occurred or worsened compared with baseline following initiation of mitapivat and up to 4 weeks after the last dose as treatment-emergent adverse events (TEAEs) and graded according to the National Cancer Institute Common Terminology Criteria for AEs (CTAE), version 5.0. All relationships of AEs to the study drug, as well as to SCD and other causes, were adjudicated by NHLBI investigators. Safety assessments included clinical history, symptom review, physical examination, vital signs (blood pressure, heart rate, and temperature), and standard clinical laboratory tests (chemistry and complete hematology panel) that were performed at the NIH Clinical Laboratories at screening, baseline, every 2 weeks at dose escalation, at the end of drug taper, and at the end of study (EOS).
Secondary end points were characterization of the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of mitapivat and clinical efficacy as assessed by changes in hemoglobin and markers of hemolysis (reticulocyte count [ARC], total bilirubin, and lactate dehydrogenase [LDH]). Other secondary end points included assessment of oxygen affinity (p50) and sickling kinetics (t50) at baseline, every 2 weeks at dose escalation, at the end of drug taper, and at EOS.
PK and PD
Serial blood collection was performed for PK and PD studies at baseline and immediately before the initial dose of each dose level; at 1, 2, 4, 8, and 12 hours after the first dose of each dose level; at the start and end of drug taper; and 4 weeks after the last dose of drug (defined as EOS). The 12-hour timepoint was removed with a protocol amendment to lower burden on patients as this avoided an additional overnight stay, and minimal differences were observed in the initial patients between the 8-hour and 12-hour timepoints. Plasma mitapivat concentrations were measured using a validated liquid chromatography tandem mass spectrometry assay following a simple protein precipitation extraction. The lower limit of quantitation was 0.500 ng/mL. Whole blood levels of ATP and 2,3-DPG were measured using liquid chromatography tandem mass spectrometry with lower limit of quantitation at 50.0 μg/mL and converted to intracellular concentrations by dividing by the hematocrit (as a fraction). All ATP and 2,3-DPG levels used in analyses were corrected for hematocrit.
Assessment of oxygen affinity and sickling kinetics
Blood collected in EDTA was processed for p50 (the single parameter marker of oxygen affinity, defined as the partial pressure of oxygen at which Hb is 50% saturated with oxygen) and t50, a single parameter measure of sickling kinetics. A Hemox Analyzer (TCS, Scientific Corp., New Hope, PA) was used to obtain p50 values. t50 is defined as the time at which 50% of erythrocytes are sickled in response to gradual deoxygenation with nitrogen to 5% oxygen.30 The half-time for the oxygen to decrease from 20% in room air to 5% at the bottom of the well plate is 40 minutes. p50 and t50 values were measured at baseline and immediately before the initial dose of each dose level, at the start and end of drug taper, and at EOS.
Statistical analysis and sample size
All consented patients were included in the primary safety analysis of AEs, stratified by severity and attribution. Patients completing at least the 50-mg BID dose level were included in the PK, PD, and efficacy analyses. Tabulations were used for demographic data, safety data, and laboratory data. Changes in clinical laboratory and PD parameters were expressed as mean differences from baseline (as either an absolute difference or a percentage change), with baseline defined as the most recent measurement prior to administration of the first dose of study drug. Mean absolute change was used for clinical variables to allow for clinical interpretation of the data, whereas mean percentage change was used for PD parameters to allow for relative changes.
The sample size was primarily driven by feasibility considerations and the ability to detect AEs. Supplemental Table 1 shows the probability of detecting at least one AE as a function of sample size and true underlying AE rate.
Statistical modeling
The P values given for the efficacy analysis of mitapivat dose effects are generated by a mixed effects regression model that provides estimates of dose level effects for a given outcome measure while adjusting for sex and age (referred to as the basic model). These regression model P values will usually (but not always) qualitatively agree with visual impressions of the changes shown in the figures. An extension of the primary regression model additionally adjusts for HU use (referred to as the extended model). Details of the modeling are provided in the Supplemental material. The P value threshold for declaring statistical significance was a 2-sided P ≤ .05; results were not adjusted for multiple comparisons.
Results
Patients
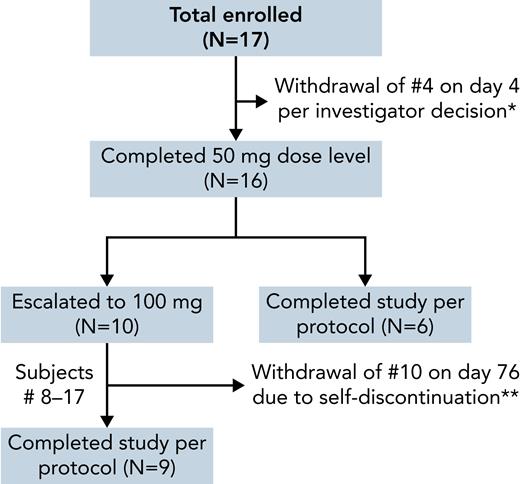
Seventeen patients were enrolled (July 2019 to March 2021), with a mean age of 39 years; 11 were male, 11 were on a stable dose of HU, and 1 was on both HU and l-glutamine (Table 1). All patients were African or African American and had a confirmed genotype of HbSS (SCD-HbSS). One patient was withdrawn on day 4 of drug treatment per investigator decision because of the need for medical interventions for a preexisting pulmonary embolism (PE; Figure 1). Of the remaining 16 patients, all successfully escalated to and completed the 50-mg dose level. Subsequent to a protocol amendment, the last 10 patients escalated to a 100-mg dose level. One patient abruptly self-discontinued treatment on day 3 of the 100-mg dose level because of an AE of pain that was attributed to a recent total hip replacement surgery and unrelated to the drug. This patient was analyzed with the 50-mg BID dose cohort (N = 7) rather than with the 100-mg BID dose cohort (N = 9), and the end of taper timepoint was not captured as the patient did not undergo a drug taper (Figure 1). The patient did not report any issues following discontinuation of drug without taper. The demographics and baseline clinical laboratory data for the patients are shown in Table 1.
Patient demographics and baseline characteristics
| . | N = 17 . |
|---|---|
| Characteristics | |
| Mean age (range), y | 39 (23-55) |
| Sex, no. (%) | |
| Female | 6 (35.3) |
| Male | 11 (64.7) |
| African or African American race, no. (%) | 17 (100) |
| Hb SS genotype, no. (%) | 17 (100) |
| Hydroxyurea use, no. (%) | 12 (70.6) |
| l-glutamine use, no. (%) | 1 (5.9) |
| Eligible for 50-mg dose level, no. | 16 |
| Eligible for 100-mg dose level, no. | 9 |
| Baseline laboratory measures | N = 16 |
| Mean hemoglobin (SD), g/dL | 9.2 (1.1) |
| Mean absolute reticulocyte count (SD), K/μL | 188.2 (99.2) |
| Mean total bilirubin (SD), mg/dL | 2.0 (0.9) |
| Mean lactate dehydrogenase (SD), U/L | 375.2 (120.6) |
| Median hemoglobin F, % (25th, 75th percentile) | 21.6 (12.4, 26.9) |
| Median ferritin (25th, 75th percentile), mcg/L | 177 (110, 318.25) |
| . | N = 17 . |
|---|---|
| Characteristics | |
| Mean age (range), y | 39 (23-55) |
| Sex, no. (%) | |
| Female | 6 (35.3) |
| Male | 11 (64.7) |
| African or African American race, no. (%) | 17 (100) |
| Hb SS genotype, no. (%) | 17 (100) |
| Hydroxyurea use, no. (%) | 12 (70.6) |
| l-glutamine use, no. (%) | 1 (5.9) |
| Eligible for 50-mg dose level, no. | 16 |
| Eligible for 100-mg dose level, no. | 9 |
| Baseline laboratory measures | N = 16 |
| Mean hemoglobin (SD), g/dL | 9.2 (1.1) |
| Mean absolute reticulocyte count (SD), K/μL | 188.2 (99.2) |
| Mean total bilirubin (SD), mg/dL | 2.0 (0.9) |
| Mean lactate dehydrogenase (SD), U/L | 375.2 (120.6) |
| Median hemoglobin F, % (25th, 75th percentile) | 21.6 (12.4, 26.9) |
| Median ferritin (25th, 75th percentile), mcg/L | 177 (110, 318.25) |
Flowchart for the dose escalation study of mitapivat in SCD. ∗Subject 4 was withdrawn because of the need for medical intervention for pulmonary embolism present unknowingly at enrollment and unrelated to drug; the subject was lost to follow-up and not evaluable for laboratory response. ∗∗Subject 10 self-discontinued therapy before completing the 100-mg dose level because of an AE unrelated to the drug; analyzed with the 50-mg dose cohort.
Flowchart for the dose escalation study of mitapivat in SCD. ∗Subject 4 was withdrawn because of the need for medical intervention for pulmonary embolism present unknowingly at enrollment and unrelated to drug; the subject was lost to follow-up and not evaluable for laboratory response. ∗∗Subject 10 self-discontinued therapy before completing the 100-mg dose level because of an AE unrelated to the drug; analyzed with the 50-mg dose cohort.
Safety and tolerability
Overall, mitapivat was well tolerated at all dose levels. No patients required a dose reduction or halting of dose escalation because of AEs. Of the 17 patients included in the safety analysis, 16 (94.1%) had at least 1 TEAE, with the majority being grade 1 or 2 in severity; with the exception of insomnia, most events were not related to drug treatment (Table 2). The most frequently reported TEAEs were insomnia (n = 7 patients, grades 1-2), hyperglycemia (n = 5, grade 1), pain (n = 5, grades 1-3), and VOC (n = 4, grade 3). Insomnia was assessed by investigators to be possibly related to treatment in 6 of 7 patients (85.7%); most (4 of 6, 66.7%) were transient and resolved within 7 days of onset of the TEAE. All hyperglycemia events were grade 1 per common terminology criteria for adverse events (abnormal glucose above baseline with no medical intervention) and occurred postprandial or in the setting of acute illness requiring hospitalization. Values ranged from 104 to 187 mg/dL. None were deemed related to treatment. Of the 5 pain TEAEs, 3 were typical of the patients’ sickle cell–related pain, did not require hospitalization, and were unlikely related to treatment. One pain AE was related to a preexisting PE, and another was shoulder pain requiring hospitalization. Other TEAEs occurring in more than 1 patient and possibly related to treatment included arthralgia (n = 3, grades 1-2), hypertension (n = 3, grades 1-3), and headache (n = 2, grades 1-2). In general, mitapivat’s safety profile is consistent with previous reports.29,31
TEAEs
| Adverse events . | All patients (N = 17) . | |
|---|---|---|
| Summary of TEAEs, no. of patients (%) | ||
| At least 1 adverse event | 16 (94.1) | |
| At least 1 adverse event of grade ≥ 3 | 7 (41.2) | |
| At least 1 serious adverse event | 6 (35.3) | |
| At least 1 serious adverse event related to treatment | 2 (11.8) | |
| Most common TEAEs, no. of events (% of patients) | All grades (≥10%) | Grade ≥3 |
| Insomnia | 7 (41.2) | 0 (0) |
| Hyperglycemia | 5 (29.4) | 0 (0) |
| Pain | 5 (29.4) | 2 (11.8) |
| Vaso-occlusive crisis | 4 (23.5) | 4 (23.5) |
| Anemia | 3 (17.6) | 2 (11.8) |
| Arthralgia | 3 (17.6) | 0 (0) |
| AST increased | 3 (17.6) | 0 (0) |
| Creatinine phosphokinase (CPK) increased | 3 (17.6) | 0 (0) |
| Headache | 3 (17.6) | 0 (0) |
| Hypertension | 3 (17.6) | 1 (5.9) |
| Hyperuricemia | 3 (17.6) | 0 (0) |
| Hyponatremia | 3 (17.6) | 0 (0) |
| Back pain | 2 (11.8) | 0 (0) |
| Blood bicarbonate decreased | 2 (11.8) | 0 (0) |
| Blood bilirubin increased | 2 (11.8) | 0 (0) |
| Diarrhea | 2 (11.8) | 0 (0) |
| Dyspnea | 2 (11.8) | 0 (0) |
| Heart rate increased | 2 (11.8) | 0 (0) |
| Helicobacter pylori infection | 2 (11.8) | 0 (0) |
| Skin ulceration | 2 (11.8) | 0 (0) |
| Sore throat | 2 (11.8) | 0 (0) |
| Upper respiratory infection | 2 (11.8) | 0 (0) |
| Serious adverse events, no. of events (% of patients) | ||
| All | 6 (35.3) | |
| VOC† | 4 (23.5) | |
| Pain (shoulder) | 1∗ (5.9) | |
| PE | 1 (5.9) | |
| Adverse events . | All patients (N = 17) . | |
|---|---|---|
| Summary of TEAEs, no. of patients (%) | ||
| At least 1 adverse event | 16 (94.1) | |
| At least 1 adverse event of grade ≥ 3 | 7 (41.2) | |
| At least 1 serious adverse event | 6 (35.3) | |
| At least 1 serious adverse event related to treatment | 2 (11.8) | |
| Most common TEAEs, no. of events (% of patients) | All grades (≥10%) | Grade ≥3 |
| Insomnia | 7 (41.2) | 0 (0) |
| Hyperglycemia | 5 (29.4) | 0 (0) |
| Pain | 5 (29.4) | 2 (11.8) |
| Vaso-occlusive crisis | 4 (23.5) | 4 (23.5) |
| Anemia | 3 (17.6) | 2 (11.8) |
| Arthralgia | 3 (17.6) | 0 (0) |
| AST increased | 3 (17.6) | 0 (0) |
| Creatinine phosphokinase (CPK) increased | 3 (17.6) | 0 (0) |
| Headache | 3 (17.6) | 0 (0) |
| Hypertension | 3 (17.6) | 1 (5.9) |
| Hyperuricemia | 3 (17.6) | 0 (0) |
| Hyponatremia | 3 (17.6) | 0 (0) |
| Back pain | 2 (11.8) | 0 (0) |
| Blood bicarbonate decreased | 2 (11.8) | 0 (0) |
| Blood bilirubin increased | 2 (11.8) | 0 (0) |
| Diarrhea | 2 (11.8) | 0 (0) |
| Dyspnea | 2 (11.8) | 0 (0) |
| Heart rate increased | 2 (11.8) | 0 (0) |
| Helicobacter pylori infection | 2 (11.8) | 0 (0) |
| Skin ulceration | 2 (11.8) | 0 (0) |
| Sore throat | 2 (11.8) | 0 (0) |
| Upper respiratory infection | 2 (11.8) | 0 (0) |
| Serious adverse events, no. of events (% of patients) | ||
| All | 6 (35.3) | |
| VOC† | 4 (23.5) | |
| Pain (shoulder) | 1∗ (5.9) | |
| PE | 1 (5.9) | |
One episode of shoulder pain, and 1 preexisting PE.
VOC is defined as acute clinical events that has no evident cause other than SCD, including acute episodes of pain requiring treatment with parenteral opioids at a medical facility, acute chest syndrome, hepatic sequestration, splenic sequestration, and priapism. Two of 4 VOCs were possibly related to the study drug, both occurred during the drug taper.
Six SAEs were reported in 6 of 17 subjects, including 4 VOCs, 1 episode of shoulder pain, and 1 preexisting PE. Of the 4 VOCs, 2 occurred during drug taper and were possibly drug related; the other 2 occurred in the last 2 days of the 28-day posttreatment period in the setting of self-reported VOC triggers (air travel, exposure to cold, and stress). No VOCs occurred during the dose escalation period. The SAE of shoulder pain was felt to be musculoskeletal and not typical of VOC symptoms but did require hospitalization and parenteral opioids for pain management. The PE was diagnosed retrospectively on computed tomography scan and required hospitalization and medical interventions that led to the withdrawal of the patient from the study per investigator decision. There were no treatment discontinuations because of AEs related to the study drug.
Nine grade 3 TEAEs occurred in 7 of 17 patients (41.2%), of which 5 were assessed as possibly related to study drug: 1 VOC each in 2 patients (discussed above), 1 episode of hypertension in 1 patient with baseline grade 2 hypertension, and the interrelated events of fatigue and anemia that occurred in a single patient following drug taper during the 28-day safety follow up. The latter AE of anemia did not require hospitalization, but the patient received a simple blood transfusion, leading to exclusion of their posttransfusion data at the end of study visit from the laboratory analysis.
Efficacy
In 16 evaluable patients, the mean hemoglobin increase was 1.2 g/dL (standard deviation [SD], 0.95 g/dL) at the 50-mg BID dose level and 0.9 g/dL (SD, 1.12 g/dL) at the 100-mg BID dose level (Figure 2; Tables 3 and 4). More than half (9 of 16, 56.3%) achieved a hemoglobin response, defined as a ≥1 g/dL increase in Hb at any dose level compared with baseline. Using a linear mixed effects model of hemoglobin change at different dose levels, with age and sex as covariates (basic model), we found a strong signal for a significant mean increase in hemoglobin level at the 20-, 50-, and 100-mg BID dose levels of mitapivat compared with baseline (Figure 2). Age and sex did not have an effect on hemoglobin response, nor did the presence of concurrent HU therapy (see supplemental Materials).
Hemoglobin response in study subjects. Mean hemoglobin response in all 16 patients completing the 50-mg BID dose level, and 9 of 10 subjects completing the 100-mg BID dose level. Error bars correspond to SDs. ∗A linear mixed effects model of hemoglobin change at different dose levels with age and sex as covariates showed a significant change in hemoglobin level compared with baseline at the 20-, 50-, and 100-mg BID dose levels of mitapivat (P = .0007, <.0001, and .0007, respectively).
Hemoglobin response in study subjects. Mean hemoglobin response in all 16 patients completing the 50-mg BID dose level, and 9 of 10 subjects completing the 100-mg BID dose level. Error bars correspond to SDs. ∗A linear mixed effects model of hemoglobin change at different dose levels with age and sex as covariates showed a significant change in hemoglobin level compared with baseline at the 20-, 50-, and 100-mg BID dose levels of mitapivat (P = .0007, <.0001, and .0007, respectively).
Absolute hemoglobin levels (g/dL) in response to mitapivat treatment
| Measurement . | N . | Mean . | SD . | 25th percentile . | Median . | 75th percentile . |
|---|---|---|---|---|---|---|
| Baseline | 16 | 9.19 | 1.10 | 8.55 | 9.55 | 9.93 |
| 5 mg BID | 16 | 9.53 | 1.22 | 8.43 | 9.80 | 10.43 |
| 20 mg BID | 16 | 9.95 | 1.28 | 9.00 | 10.00 | 10.83 |
| 50 mg BID | 16 | 10.38 | 1.21 | 9.52 | 10.15 | 11.25 |
| 100 mg BID | 9 | 10.06 | 1.00 | 9.30 | 10.30 | 10.80 |
| End of taper | 15 | 9.43 | 1.24 | 8.70 | 9.60 | 10.40 |
| End of study | 15 | 9.64 | 1.33 | 8.65 | 9.60 | 10.60 |
| Measurement . | N . | Mean . | SD . | 25th percentile . | Median . | 75th percentile . |
|---|---|---|---|---|---|---|
| Baseline | 16 | 9.19 | 1.10 | 8.55 | 9.55 | 9.93 |
| 5 mg BID | 16 | 9.53 | 1.22 | 8.43 | 9.80 | 10.43 |
| 20 mg BID | 16 | 9.95 | 1.28 | 9.00 | 10.00 | 10.83 |
| 50 mg BID | 16 | 10.38 | 1.21 | 9.52 | 10.15 | 11.25 |
| 100 mg BID | 9 | 10.06 | 1.00 | 9.30 | 10.30 | 10.80 |
| End of taper | 15 | 9.43 | 1.24 | 8.70 | 9.60 | 10.40 |
| End of study | 15 | 9.64 | 1.33 | 8.65 | 9.60 | 10.60 |
Change in hemoglobin levels (g/dL) in response to mitapivat treatment
| Measurement . | N . | Mean . | SD . | 25th percentile . | Median . | 75th percentile . |
|---|---|---|---|---|---|---|
| Baseline | 16 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 |
| 5 mg BID | 16 | 0.34 | 0.70 | −0.12 | 0.3 | 0.80 |
| 20 mg BID | 16 | 0.76 | 0.96 | −0.10 | 0.7 | 1.30 |
| 50 mg BID | 16 | 1.19 | 0.95 | 0.57 | 1.1 | 1.65 |
| 100 mg BID | 9 | 0.87 | 1.12 | 0.40 | 0.8 | 0.90 |
| End of taper | 15 | 0.29 | 0.82 | −0.20 | 0.3 | 0.55 |
| End of study | 15 | 0.40 | 0.69 | 0.05 | 0.6 | 0.90 |
| Measurement . | N . | Mean . | SD . | 25th percentile . | Median . | 75th percentile . |
|---|---|---|---|---|---|---|
| Baseline | 16 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 |
| 5 mg BID | 16 | 0.34 | 0.70 | −0.12 | 0.3 | 0.80 |
| 20 mg BID | 16 | 0.76 | 0.96 | −0.10 | 0.7 | 1.30 |
| 50 mg BID | 16 | 1.19 | 0.95 | 0.57 | 1.1 | 1.65 |
| 100 mg BID | 9 | 0.87 | 1.12 | 0.40 | 0.8 | 0.90 |
| End of taper | 15 | 0.29 | 0.82 | −0.20 | 0.3 | 0.55 |
| End of study | 15 | 0.40 | 0.69 | 0.05 | 0.6 | 0.90 |
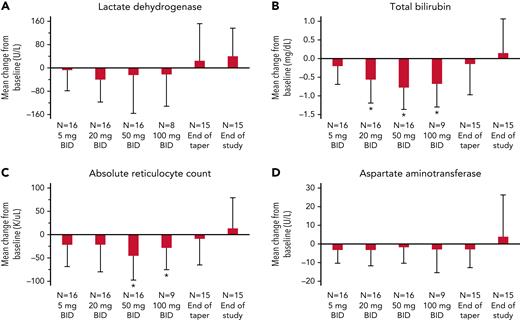
An absolute mean reduction in all hemolytic markers, LDH, total serum bilirubin, ARC, and aspartate aminotransferase (AST) was noted during the dose escalation period, although individual responses were variable, as reflected by the large SDs (Figure 3A-D). The basic model also showed a strong effect of mitapivat treatment on total bilirubin levels that mirrored the hemoglobin response, with a significant reduction at the 20-, 50-, and 100-mg BID dose levels (Figure 3B). A weaker effect was seen for ARC reduction at the 50- and 100-mg BID dose levels (Figure 3C). No significant reductions in LDH (Figure 3A) and AST (Figure 3D) were observed.
Change in hemolysis markers from baseline in study subjects. Hemolysis markers, (A) LDH, (B) total bilirubin, (C) ARC, and (D) AST, were measured at steady state (baseline); after 14 ± 3 days of treatment on 5 mg BID, 20 mg BID, 50 mg BID, and 100 mg BID of mitapivat; at the end of taper (1 ± 3 days after the last dose of mitapivat); and at EOS (4 weeks ± 3 days after the last dose of mitapivat). A total of 16 subjects escalated to the 50-mg BID dose, and 9 of 10 subjects completed up to the 100-mg BID dose level. End of taper data were unavailable for 1 subject who self-discontinued treatment without undergoing a taper; EOS data were excluded for 1 subject because of intervening blood transfusion. Mean change was calculated from baseline, defined as the most recent measurement prior to start of study drug and reported as absolute change. Error bars correspond to SDs. A linear mixed effects model using age and sex as covariates was created for each variable (referred to as the basic model; supplemental Materials). No significant changes from baseline were identified for LDH (A) and AST (D). ∗There was a significant absolute reduction in (B) total bilirubin level compared with baseline at the 20-, 50-, and 100-mg BID dose levels of mitapivat (P = .001, <.0001, and <.0001, respectively). (C) Reduction in ARC compared with baseline was significant at the 50- and 100-mg BID dose levels of mitapivat (P = .002 and .05, respectively), as denoted by ∗.
Change in hemolysis markers from baseline in study subjects. Hemolysis markers, (A) LDH, (B) total bilirubin, (C) ARC, and (D) AST, were measured at steady state (baseline); after 14 ± 3 days of treatment on 5 mg BID, 20 mg BID, 50 mg BID, and 100 mg BID of mitapivat; at the end of taper (1 ± 3 days after the last dose of mitapivat); and at EOS (4 weeks ± 3 days after the last dose of mitapivat). A total of 16 subjects escalated to the 50-mg BID dose, and 9 of 10 subjects completed up to the 100-mg BID dose level. End of taper data were unavailable for 1 subject who self-discontinued treatment without undergoing a taper; EOS data were excluded for 1 subject because of intervening blood transfusion. Mean change was calculated from baseline, defined as the most recent measurement prior to start of study drug and reported as absolute change. Error bars correspond to SDs. A linear mixed effects model using age and sex as covariates was created for each variable (referred to as the basic model; supplemental Materials). No significant changes from baseline were identified for LDH (A) and AST (D). ∗There was a significant absolute reduction in (B) total bilirubin level compared with baseline at the 20-, 50-, and 100-mg BID dose levels of mitapivat (P = .001, <.0001, and <.0001, respectively). (C) Reduction in ARC compared with baseline was significant at the 50- and 100-mg BID dose levels of mitapivat (P = .002 and .05, respectively), as denoted by ∗.
Additionally, there was a borderline increase in mean corpuscular volume (MCV) at the 100-mg dose level (P = .05) and small decreases in mean HbF levels at the 20- and 50-mg dose levels (P = .04 and .009, respectively; basic model, supplemental Materials). Two patients were noted to have down trending MCVs during the study and were diagnosed with and treated for Helicobacter pylori infection and oral iron supplementation for iron deficiency. As regression analysis showed that the Hb response in the entire cohort with and without the 2 iron-deficient individuals do not change qualitatively, we have included these 2 individuals in the study to be consistent with the principles of including all the data to the extent possible to enhance generalizability to a broad SCD population and avoid second-guessing or potential criticism about why some people may be excluded. Both these iron-deficient individuals were not on HU. No modifications were made to concurrent HU or l-glutamine therapy during the study period.
PK and PD
The PKs of mitapivat were evaluated in subjects with SCD for the first time in this study (refer to supplemental Materials for details). The PKs of mitapivat in this study were consistent with those previously observed in healthy adults.27,28
A dose-dependent decrease in mean 2,3-DPG levels and dose-dependent increase in mean ATP levels were observed following multiple ascending doses of mitapivat (Figure 4A-B), followed by a return toward baseline by EOS. Regression modeling showed a pronounced response in both PD parameters at the 20-, 50-, and 100-mg BID dose levels in the basic model (Figure 4A-B; supplemental Material). There was also a mean percentage decrease in p50 and increase in t50 at all dose levels, although these changes were not significant in the basic model (Figure 4C-D; supplemental Material).
Change in 2,3-DPG, ATP, p50, and t50 from baseline in study subjects. Measures of PD, (A) 2,3-DPG, (B) ATP, (C) p50, and (D) t50, were assessed at steady state (baseline); after 14 ± 3 days of treatment on 5 mg BID, 20 mg BID, 50 mg BID, and 100 mg BID of mitapivat; at the end of taper (1 ± 3 days after the last dose of mitapivat); and at EOS (4 weeks ± 3 days after the last dose of mitapivat). A total of 16 subjects escalated to the 50-mg BID dose, and 9 of 10 subjects completed up to the 100-mg BID dose level. End of taper data were unavailable for 1 subject who self-discontinued treatment without undergoing a taper; EOS data were excluded for 1 subject because of intervening blood transfusion. Missing data reflected in the sample sizes shown (in particular, for p50) were largely the result of disruptions related to the COVID-19 pandemic. Mean change was calculated from baseline, defined as the most recent measurement prior to start of study drug, and reported as percentage change for ease of clinical interpretation. Error bars correspond to SDs. A linear mixed effects model using age and sex as covariates was created for each variable (referred to as the basic model; supplemental Materials), and statistical significance within this model is denoted in the figures by ∗. (A-B) There was a significant mean percentage decrease in 2,3-DPG levels and mean percentage increase in ATP levels compared with baseline at the 20-, 50-, and 100-mg BID dose levels of mitapivat (P < .0001 for all). (C-D) No significant changes from baseline were identified for p50 and t50 values in the basic model, except for a percentage increase p50 at the end-of-taper and end-of-study timepoints (P = .02 and .003, respectively).
Change in 2,3-DPG, ATP, p50, and t50 from baseline in study subjects. Measures of PD, (A) 2,3-DPG, (B) ATP, (C) p50, and (D) t50, were assessed at steady state (baseline); after 14 ± 3 days of treatment on 5 mg BID, 20 mg BID, 50 mg BID, and 100 mg BID of mitapivat; at the end of taper (1 ± 3 days after the last dose of mitapivat); and at EOS (4 weeks ± 3 days after the last dose of mitapivat). A total of 16 subjects escalated to the 50-mg BID dose, and 9 of 10 subjects completed up to the 100-mg BID dose level. End of taper data were unavailable for 1 subject who self-discontinued treatment without undergoing a taper; EOS data were excluded for 1 subject because of intervening blood transfusion. Missing data reflected in the sample sizes shown (in particular, for p50) were largely the result of disruptions related to the COVID-19 pandemic. Mean change was calculated from baseline, defined as the most recent measurement prior to start of study drug, and reported as percentage change for ease of clinical interpretation. Error bars correspond to SDs. A linear mixed effects model using age and sex as covariates was created for each variable (referred to as the basic model; supplemental Materials), and statistical significance within this model is denoted in the figures by ∗. (A-B) There was a significant mean percentage decrease in 2,3-DPG levels and mean percentage increase in ATP levels compared with baseline at the 20-, 50-, and 100-mg BID dose levels of mitapivat (P < .0001 for all). (C-D) No significant changes from baseline were identified for p50 and t50 values in the basic model, except for a percentage increase p50 at the end-of-taper and end-of-study timepoints (P = .02 and .003, respectively).
HU use was associated with lower ARC, higher MCV, higher HbF percentage, a greater change in ATP levels, and a smaller change in t50 (supplemental Materials). For most variables, the extended model did not differ notably from the basic model. However, after controlling for HU, mean percentage increases in t50 were found to be significant at the 20-, 50-, and 100-mg dose levels of mitapivat (P = .005, .01, and 01, respectively; supplemental Materials).
Discussion
Treatment with mitapivat during a 6- to 8-week period in patients with SCD-HbSS demonstrated an acceptable safety and tolerability profile, consistent with prior experience in the studies of patients with PKR deficiency and thalassemia. Patients tolerated all 4 dose levels (5, 20, 50, and 100 mg BID) well, with no treatment discontinuations for drug-related AEs or drug intolerance. In total, 15 patients completed treatment per protocol, and 2 patients discontinued treatment because of reasons unrelated to drug treatment. Additionally, there was improvement in hemolytic anemia at multiple ascending dose levels in patients with SCD.
The majority of TEAEs were grade 1 and 2 and unrelated to study drug. A total of 5 grade 3 TEAEs related to study drug occurred in 17 patients. None occurred during the dose escalation period, and 4 of 5 (2 VOCs and a concurrent episode of anemia and fatigue) occurred during the drug taper period, suggesting a possible relationship to withdrawal of the drug’s salutary effects rather than to adverse effects of the drug itself. In the phase 2 study of mitapivat for pyruvate kinase deficiency, acute hemolysis was identified in 2 patients after abrupt cessation of mitapivat at 300-mg dosing, leading to implementation of a drug taper. By contrast, subjects who missed only a few doses of mitapivat, or for whom the dose was tapered, did not experience events indicative of acute hemolysis.29 The other grade 3 TEAE of hypertension occurred in a patient with baseline grade 2 hypertension and was possibly related to physiologic fluctuations in vital signs throughout the day. Increases in blood pressure and heart rate were also detected in other patients in the setting of frequent nursing assessments during 12-hour inpatient stays for PK blood draws. In addition, 2 other VOCs occurred when the patients were not on study drug (1 occurred 4 weeks after last dose of study drug); the VOCs occurred in the setting of typical self-reported VOC triggers and were considered unrelated to drug. VOCs are complications not unexpected in the patients with SCD; the short treatment duration of this dose escalation study does not allow for an assessment of mitapivat’s effect on VOC frequency.
More than half (9 of 16) of the patients with SCD treated with mitapivat experienced an increase in hemoglobin level of ≥1 g/dL, with statistically significant increases in hemoglobin above baseline observed starting at the 20-mg BID dose. The mean peak hemoglobin response was reached after 2 weeks of treatment on the 50-mg BID dose. A recent meta-analysis found a reduction in the risk of stroke/silent cerebral infarcts, albuminuria, elevated pulmonary artery systolic pressure, and mortality by 41%, 53%, 57%, and 64%, respectively, for every 1-g/dL increase in hemoglobin level,32 suggesting mitapivat has the potential to improve clinical outcomes by raising hemoglobin levels. The improvements in markers of hemolysis support the idea that reduced sickling and maintenance of RBC integrity underlies the Hb increase.
Concerns have been raised with increasing hemoglobin level in patients with SCD above 10 g/dL because of anecdotal reports of viscosity-related neurologic complications in patients transfused to a hemoglobin > 10 g/dL for acute complications.33 In a phase 3 study of senicapoc (a Gardos channel inhibitor), hemolytic anemia was reduced but a higher rate of VOC was detected in the treatment arm, raising the possibility that increasing hemoglobin level may lead to increased blood viscosity and vaso-occlusion.34 A recent re-analysis, however, confirmed that the sickle cell pain rate in all subjects with Hb response was not significantly different from those observed in the placebo group.35 The risk of viscosity-related adverse effects is likely multifactorial, and although closely related to hematocrit, this relationship can be modified by the mechanism of therapeutic increase that has been shown by HU therapy.33 In this study, the highest hemoglobin level reached was 12.6 g/dL, and no issues were reported.
No vaso-occlusive events or hyperviscosity-associated symptoms were reported while patients were undergoing dose escalation despite hemoglobin increases of as much as 3.4 g/dL from baseline, suggesting that mitapivat may be able to safely increase hemoglobin levels in patients with SCD. We propose that mitapivat may offset the effect of raising hemoglobin levels with its anti-sickling effects. The reductions in 2,3-DPG levels are translated in the decreased p50 and reduced sickling kinetics. Furthermore, mitapivat exposure was also associated with a dose-dependent increase in levels of RBC ATP, a key component for maintaining RBC integrity that reduces hemolysis. ATP also increases RBC hydration, and although, we did not perform rheological and deformability or viscosity studies, mitapivat therapy is associated with an improvement in sickling kinetics indicated by the t50 parameters. A recent in vitro study using the Laser Optical Rotational Red Cell Analyzer (LORRCA; RR Mechatronics) and oxygen gradient ektacytometry to assess sickling kinetics also showed that mitapivat improved the point of sickling and deformability (measured as Elmax) of HbSS RBCs.36
We noted a small decrease in mean HbF percentage with mitapivat treatment that is open to interpretation given the small study and missing datapoints in some subjects. The modifying effect of background HU therapy also needs a larger study with a larger sample size of on-and-off HU therapy subjects for any meaningful interpretation.
The US Food and Drug Administration approval of l-glutamine (Endari; Emmaus Medical, Inc, Torrance, CA),37 crizanlizumab (Adakveo; Novartis Pharmaceuticals Corporation, East Hanover, NJ),38 and voxelotor (Oxbryta; Global Blood Therapeutics, Inc, South San Francisco, CA)39 within a short timeframe marks the beginning of an era of SCD targeted therapies. Of these newly approved drugs, voxelotor is the only agent that directly targets HbS polymerization by increasing hemoglobin oxygen affinity.40 An extended study of voxelotor supports the hemoglobin increase via amelioration of hemolysis, but there remains no reduction of painful episodes except in a small fraction of patients with SCD with hemoglobin levels above 12 g/dL.17,18,41
In this study, we provided proof of concept for activating PKR in SCD; the results suggest that activating PKR is a viable therapeutic approach in SCD. Mitapivat, a PKR activator, improved anemia, reduced markers of hemolysis, decreased 2,3-DPG and increased ATP levels, increased oxygen affinity, and reduced sickling. The long-term safety profile of mitapivat and its potential to reduce VOCs and pain are currently being evaluated in an ongoing extended treatment study (#NCT04610866). Additionally, results from this study informed the design of an ongoing phase 2/3 trial of mitapivat in patients with SCD (RISE UP; #NCT05031780), evaluating both improvements in Hb and frequency of sickle cell pain crises. This phase 2/3 randomized controlled trial will also evaluate the safety of mitapivat during the core and follow-up periods of the study.
Acknowledgments
This work was supported by the Division of Intramural Research of the National Heart, Lung, and Blood Institute and National Institute of Diabetes, and Digestive and Kidney Disease, National Institutes of Health. Mitapivat was provided by Agios.
Authorship
Contribution: S.L.T. was the principal investigator, wrote the initial clinical trial protocol, and supervised the study; J.Z.X., A.C., I.F., E.G., L.A.M., and S.L.T. recruited and enrolled patients, determined attributions for adverse events, and wrote subsequent protocol amendments; L.T., M.L., and T.L. collected and processed blood samples for PK and PD studies; Q.L., K.G., and E.B.D. performed experiments to measure p50 and t50; PK, 2,3-DPG, and ATP data were generated by QPS in partnership with Agios Pharmaceuticals; V.I., H.M., C.K., L.D., and P.A.K. oversaw the data generation and provided input in the analysis of these data; P.H. provided input in adverse event attributions and the safety data analysis; W.A.E. oversaw p50 and t50 data collection and provided input in the PD data analysis and overall study design; N.J. performed the statistical analysis; J.Z.X. and S.L.T. oversaw the data collection, cleaning, and analysis; J.Z.X. wrote the first draft of the manuscript; J.Z.X., S.L.T., and N.J. reviewed and wrote subsequent drafts that were reviewed by all authors; and the final version of the manuscript was approved by all authors for publication.
Conflict-of-interest disclosure: V.I., H.M., C.K., L.D., P.A.K., and P.H. are employed by and are stockholders in Agios. H.M. and P.H. are stockholders in Bristol-Myers Squibb. V.I. is a stockholder in Novartis. The remaining authors declare no competing financial interests.
Correspondence: Swee Lay Thein, National Heart, Lung and Blood Institute/National Institutes of Health, Bldg 10-CRC, Room 6S241, 10 Center Dr, Bethesda, MD 20892; e-mail: sl.thein@nih.gov.
References
Author notes
Deidentified individual participant data will be made available 6 months after publication date for a period of 5 years by sending a request to the corresponding author at sweelay.thein@nih.gov.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal