Abstract
Diffuse large B-cell lymphoma (DLBCL) is a heterogenous subtype of non-Hodgkin lymphoma. Relapsed/refractory disease represents remains an unmet medical need, despite the introduction of novel cellular and targeted therapies. Loncastuximab tesirine is a cluster of differentiation19-targeting antibody-drug conjugate approved by the US Food and Drug Administration for relapsed DLBCL after 2 lines of systemic therapy based on a trial showing a 48.3% overall response rate. The spectrum of its clinical applications is expanding and is now being tested in other B-cell malignancies.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL), accounting for 30% to 40% of all newly diagnosed NHL worldwide.1 About 60% of DLBCL patients will be cured with initial standard chemo-immunotherapy (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone [R-CHOP]). However, 10% to 15% of patients treated with R-CHOP have primary refractory disease and an additional 20% to 25% will have a relapse after an initial response.2 Salvage therapy including high-dose chemotherapy and autologous stem cell transplantation can be an effective treatment for DLBCL with chemotherapy-sensitive relapse. However, more than one-half of the patients will not have long-term disease control, and a significant proportion of patients are not eligible for aggressive treatment.3
Patients who do not achieve a response to second-line treatment or who relapse after high-dose therapy and autologous stem cell transplantation had limited options before the approval of cluster of differentiation 19 (CD19)-specific chimeric antigen receptor (CAR) T-cell therapy, which allows to achieve durable complete response rates of ∼30% to 40%.4-6 However, prognosis for patients who display an unsatisfactory response to CAR T cells or experience disease relapse remains poor.7 In addition, several novel immunotherapies and targeted therapies have been approved for use in relapsed or refractory (r/r) DLBCL, as monotherapy agents or in combination with chemotherapy, with encouraging results.8-10
However, most of these studies included few patients with adverse prognostic factors. Further, an effective therapy is still lacking for certain subgroups such as elderly patients, those with biological markers of aggressiveness (double-hit/triple-hit), and those failing CAR T therapy and/or other novel agents. CD19-targeting antibody-drug conjugates (ADCs) have the potential to improve the clinical outcomes of these patients.
The CD19 antigen as a therapeutic target
The surface glycoprotein CD19 is a B cell–specific member of the immunoglobulin superfamily expressed during most stages of lymphopoiesis, from immunoglobulin gene rearrangement (pre-B-cell stage) to terminal differentiation into plasma cells.11 CD19 is crucial for the development and activation of B cells because it is involved in B-cell receptor–dependent and independent signaling.12-17 Moreover, CD19 expression and interaction with the B-cell receptor is necessary for B-cell differentiation, including early events occurring in the marrow and late events in the spleen and secondary lymphoid tissues.18-20
Most B-cell malignancies retain expression of CD19, although its role in their development is still unclear. It has been suggested that CD19 expression promotes the upregulation of MYC, a potent oncogenic protein in aggressive B-cell lymphomas.21
Several characteristics make the CD19 antigen a very attractive therapeutic target. First, its expression is specific to B cells and B-cell malignancies, with no expression in hematopoietic stem cells. Second, it is not present as a soluble, circulating isoform, allowing for the drug to be delivered safely to target cells without competitive binding. Third, it has rapid rates of internalization on antibody binding and reexpression.
Autologous CAR T cells and bispecific T-cell engagers represent 2 successful means of targeting the CD19 antigen and have been approved by the US Food and Drug Administration (FDA) for the treatment of different types of NHL and B-cell acute lymphoblastic leukemia.4-6,22 Additionally, the combination of lenalidomide and the Fc-modified anti-CD19 monoclonal antibody tafasitamab has been granted accelerated approval by the FDA for the treatment of r/r DLBCL after 1 line of therapy.8 These results suggest that targeting CD19, in particular with combined strategies, can become the cornerstone of targeted therapy for aggressive lymphomas.
CD19-targeting ADCs with a special focus on loncastuximab tesirine
ADCs are immunoconjugates comprising an engineered monoclonal antibody chemically attached to a cytotoxic drug (the payload) via a stable chemical linker.23 The result is a molecule capable of precise delivery of a cytotoxic drug to the desired target cell, resulting in an enhanced on-tumor effect, while minimizing off-target activity. Before engagement on the target cell, the conjugated monoclonal antibody circulates in the bloodstream, neither releasing its cytotoxic payload into circulation nor binding to nontarget cells. On antigen binding, the ADC is internalized via receptor-mediated endocytosis, followed by lysosomal degradation of the linker and release of the cytotoxic molecule.24 Cell death then occurs through direct DNA damage by the payload or through disruption of cellular processes such as tubulin polymerization and polypeptide synthesis.25 Seven ADCs have been approved for clinical use in various hematological disorders and have paved the way for the development of novel targets as well as the technological upgrade of the structure of the ADC itself.10,26-32
Common payloads include auristatin derivatives, calicheamicin, indolinobenzodiazepines, and duocarmycins.33 More recently, pyrrolobenzodiazepines (PBDs) have been successfully tested in numerous preclinical and clinical studies. PBDs are interstrand minor groove DNA cross-linking agents (Figure 1), which present numerous advantages compared with other cytotoxic payloads. First, PBDs do not distort the structure of DNA and can therefore effectively evade mechanisms of DNA repair.34 This allows for a prolonged cytotoxic activity to also eradicate slowly replicating tumor cells, which harbor clones that may promote disease resistance/recurrence. Second, PBDs maintain their cytotoxic activity in tumor cell lines expressing multidrug resistant proteins.35 Last, PBD-carrying ADCs exhibit a shorter half-life compared with other ADCs, thus limiting off-target activity as well as systemic toxicity.35
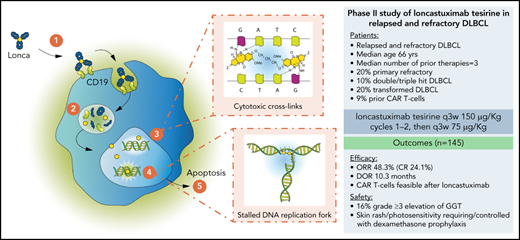
Mechanism of action of PBD-ADCs (loncastuximab tesirine [Lonca]). (1) Lonca binds to CD19 on the tumor cell surface; (2) following internalization of Lonca, the protease-sensitive linker is cleaved and cytotoxic PBD dimers are released inside the cell; (3) the free PBD dimers bind in the minor groove of the cell DNA and form potent cytotoxic DNA cross-links in a sequence- selective fashion; (4) the cross-links result in a stalled DNA replication fork, blocking cell division; and (5) the cancer cell undergoes apoptosis.
Mechanism of action of PBD-ADCs (loncastuximab tesirine [Lonca]). (1) Lonca binds to CD19 on the tumor cell surface; (2) following internalization of Lonca, the protease-sensitive linker is cleaved and cytotoxic PBD dimers are released inside the cell; (3) the free PBD dimers bind in the minor groove of the cell DNA and form potent cytotoxic DNA cross-links in a sequence- selective fashion; (4) the cross-links result in a stalled DNA replication fork, blocking cell division; and (5) the cancer cell undergoes apoptosis.
Zammarchi et al were the first to document the therapeutic potential of the anti-CD19 ADC ADCT-402 (loncastuximab tesirine) containing the PBD dimer payload SG3199, in preclinical models. In vitro, loncastuximab tesirine had a specific activity for CD19+ cells, and its killing activity was positively correlated to CD19 surface expression.36 Interestingly, also CD19− cell lines exposed to loncastuximab tesirine exhibited reduced survival, likely because of a bystander effect that may be exploited to target tumor cells with negative or variable CD19 expression.
Studies of loncastuximab tesirine in relapsed NHL
A first-in-human phase 1 study was designed to include patients with r/r NHL.37,38 Most patients were affected by DLBCL (71.6%) and had received a median of 3 lines of therapy before enrollment. Study participants were exposed to increasing doses (15 µg/kg-200 µg/kg) of loncastuximab tesirine as intravenous injection every 3 weeks. Optimal dosing was set at 150 µg/kg, which was sufficient to obtain significant antitumor activity while avoiding excessive drug accumulation and toxicity. At doses higher than 120 µg/kg, 55% of DLBCL showed an objective response. No significant immunogenicity was observed.
Based on pharmacokinetic data form the phase 1 trial, patients enrolled in the subsequent phase 2 study were administered loncastuximab tesirine at a dose of 150 µg/kg for the first 2 cycles, and at a dose of 75 µg/kg thereafter. In terms of efficacy, the phase 2 study confirmed previous encouraging data, and loncastuximab tesirine was recently granted accelerated approval from the FDA for the treatment of r/r DLBCL after 2 or more lines of systemic therapy39 (Table 1). Approval was based on achieving an objective response rate (ORR) of 48.3%, with a complete response rate of 24.1% and a median duration of response of 10.3 months.32 Most responses were achieved within the first imaging assessment (after 2 cycles of therapy), with a median time to response of 41 days. The study included patients with r/r DLBCL after 2 or more multiagent systemic treatments, of whom 32% had received at least 3 prior lines of therapy. Subgroup analyses were limited by the small sample; however, similar responses were obtained also in high-risk patients, including double- and triple-hit lymphoma, primary refractory DLBCL, and transformed DLBCL from indolent disease. Further, 13 patients (9%) who relapsed after CAR T-cell therapy were treated with loncastuximab tesirine and exhibited an overall response rate of 46%. These findings support a more extended use in relapsed DLBCL and in clinical contexts in which rapid disease control is required.
Summary of phase 1 and 2 studies of loncastuximab tesirine in r/r DLBCL
| Parameter . | Phase 1* . | Phase 2 . |
|---|---|---|
| No. of patients | 139 | 145 |
| Median age, y | 63 | 66 |
| Double/triple hit, no. | 23 (17%) | 15 (10%) |
| Transformed DLBCL | 37 (27%) | 29 (20%) |
| Median number of prior therapies, no. (range) | 3 (1-10) | 3 (2-4) |
| Prior auto-SCT, no. | 22 (16%) | 21 (14%) |
| Prior CAR T cells, no. | 2 (1%) | 13 (9%) |
| ORR | 42.3% | 48.3% |
| Complete remission | 23.4% | 24.1% |
| Median duration of response, mo | 4.5 (not reached for complete responders) | 10.3 (13.4 mo for complete responders) |
| Median PFS, mo | 2.8 | 4.9 |
| Median OS, mo | 7.5 | 9.9 |
| Time to response, d | 43 | 41 |
| Stopped drug from adverse event, no. | 35 (19%) | 34 (23%) |
| Grade ≥ 3 neutropenia, no. | 71 (40%) | 37 (26%) |
| Grade ≥ 3 pleural effusions, no. | 7 (4%) | 3 (2%) |
| Rash, no. | 45 (24.6%) | 19 (13%), only 1 case grade 3 |
| Grade ≥ 3 elevation of GGT, no. | 39 (21.3%) | 24 (16%) |
| Parameter . | Phase 1* . | Phase 2 . |
|---|---|---|
| No. of patients | 139 | 145 |
| Median age, y | 63 | 66 |
| Double/triple hit, no. | 23 (17%) | 15 (10%) |
| Transformed DLBCL | 37 (27%) | 29 (20%) |
| Median number of prior therapies, no. (range) | 3 (1-10) | 3 (2-4) |
| Prior auto-SCT, no. | 22 (16%) | 21 (14%) |
| Prior CAR T cells, no. | 2 (1%) | 13 (9%) |
| ORR | 42.3% | 48.3% |
| Complete remission | 23.4% | 24.1% |
| Median duration of response, mo | 4.5 (not reached for complete responders) | 10.3 (13.4 mo for complete responders) |
| Median PFS, mo | 2.8 | 4.9 |
| Median OS, mo | 7.5 | 9.9 |
| Time to response, d | 43 | 41 |
| Stopped drug from adverse event, no. | 35 (19%) | 34 (23%) |
| Grade ≥ 3 neutropenia, no. | 71 (40%) | 37 (26%) |
| Grade ≥ 3 pleural effusions, no. | 7 (4%) | 3 (2%) |
| Rash, no. | 45 (24.6%) | 19 (13%), only 1 case grade 3 |
| Grade ≥ 3 elevation of GGT, no. | 39 (21.3%) | 24 (16%) |
OS, overall survival; PFS, progression-free survival; SCT, stem cell transplant.
Only the DLBCL cohort was considered for patient characteristics and outcomes; safety analysis refers to all patients included in the study.
Because of the paucity of immunoconjugates currently in use, there are no data regarding mechanisms of resistance to ADCs causing clinical failure. CD19 antigen loss is an important mechanism of resistance to anti-CD19 CAR T-cell therapy, occurring in up to 30% of cases.40 Interestingly, available data on a small cohort of patients exposed to loncastuximab tesirine before anti-CD19 CAR T-cell therapy suggest that antigen loss may not be as common.41
Toxicity profile
Data from safety analysis showed that all patients experienced treatment emergent adverse events, which mainly included hematologic toxicity, fatigue, nausea, and rash. Febrile neutropenia was uncommon (grade ≥ 3 = 3%). Most common grade ≥ 3 adverse events included neutropenia (26%), thrombocytopenia (18%), and elevation of γ-glutamyl transpeptidase (GGT) (16%) in the absence of other signs of liver toxicity. Grade ≥ 3 hematological and liver toxicity can be managed by holding the drug until resolution and subsequent dose reductions by 50% if a prolonged toxicity occurs (>3 weeks). Contrary to other ADCs, peripheral neuropathy was not a common adverse event. Fluid retention, including pleural effusions (all-grade = 8%, grade ≥ 3 = 2%), peripheral edema (all grade = 19%, grade ≥ 3 = 1%), and pericardial effusion (n = 1) were observed in the phase 1 trial,37 and were effectively reduced by including premedication with oral dexamethasone (4 mg twice a day, from day −1 to +1) and spironolactone diuretics. Presumably, they are related to the PBD payload, although the pathogenetic mechanisms are still unclear and may be associated with direct vascular toxicity.42-45 Rash was also a common adverse event and occurred mainly in sun-exposed areas (12%, only 1 grade 3 case), prompting physicians to recommend avoidance of prolonged sun exposure. Of note, no cases of tumor lysis syndrome or tumor flare occurred.
The drug was discontinued because of treatment-emergent adverse effects in 23% of patients, whereas dose delays were generally short-lived (<1 week).32 Most discontinuations were due to grade ≥ 3 elevation of GGT (10%). The rate of discontinuation, albeit not negligible, is comparable to that of other novel therapies, including tafasitamab and lenalidomide (22%)8 and polatuzumab vedotin combined with chemoimmunotherapy (21%),46 although considerably higher than bispecific antibodies (0%-4%).47-49 Most importantly, especially when considering the demographics of DLBCL, the Loncastuximab Tesirine in Relapsed or Refractory Diffuse Large B-cell Lymphoma-2 trial did not reveal toxicity concerns for patients aged ≥ 65 years, including 14% of patients older than 75 years.32
Clinical scenarios for future applications of loncastuximab tesirine
Loncastuximab tesirine is the most recent addition to the ADC armamentarium for NHL therapies. Its applications are potentially broad, and is, therefore, currently being tested in other clinical settings as well as in other subtypes of NHL (NCT03684694, NCT04998669, NCT04384484; www.clinicaltrials.gov). Whereas the anti-CD79b ADC polatuzumab vedotin is a strong candidate for the treatment of transplant-ineligible r/r DLBCL in combination with chemoimmunotherapy, loncastuximab tesirine may address additional unmet clinical scenarios.
Among its attractive features, it displays a rapid action, and, in contrast to other novel immunotherapies and cellular therapies, does not appear to cause tumor flare or tumor lysis syndrome, allowing for a safe outpatient administration. Thus, it may be used sequentially or in combination with either lower doses or reduced cycles of chemotherapeutic agents, in elderly and/or unfit patients, to maximize treatment effectiveness. Further, a sequential strategy of ADC debulking therapy followed by chemotherapy may be appropriate for patients presenting with rapidly progressing disease.
DLBCL is a heterogenous disease in terms of clinical features, morphology, immunohistochemistry, and genetic defects. High-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6 (HGBCL-DH/TH) is clinically aggressive, with poor prognosis.50 Likewise, double and triple expressor DLBCL, as well as DLBCL transformed from indolent lymphoma or harboring a TP53 mutation/deletion are associated with poor outcomes.51-53 In such subgroups, standard therapy has inferior efficacy, and it is urgent to identify effective, well-tolerated treatment alternatives. In this context, loncastuximab tesirine may be a promising addition to standard regimens; indeed, a clinical trial investigating the effects of R-CHOP and loncastuximab will soon be open to enrollment (NCT04974996; www.clinicaltrials.gov).
Patients who progress on or relapse after anti-CD19 CAR T-cell therapy are left with no validated treatment alternatives and often exhibit a rapidly progressing disease requiring timely treatment. The efficacy of loncastuximab tesirine in this scenario has been validated in a small cohort of patients within the Loncastuximab Tesirine in Relapsed or Refractory Diffuse Large B-cell Lymphoma-2 trial.54 Patients who have CD19 persistence should still be susceptible to the direct cytotoxic effect of loncastuximab, which may also overcome immune exhaustion and possibly microenvironmental signals leading to CAR T-cell failure.40 Moreover, whereas changes in antigen density are known to affect CAR-T cell activity,40 the bystander effect observed with loncastuximab suggests this agent is much less dependent on high CD19 surface expression and may be effective also in cases with low/absent CD19 expression. Last, we can speculate that the CD19 antigen-binding domain FMC63, the target of chimeric T cells, is distinct from that of loncastuximab tesirine, B4. Thus, ADC and CAR T-cell activities should not be mutually exclusive. A trial specifically designed to include patients failing CAR T cells will be shortly open to accrual and will also address the issue of feasibility for patients recovering from CAR T-cell toxicities, such as peripheral cytopenias. Encouragingly, recent data have shown that the proportion of patients failing CAR T and being able to receive subsequent therapy is growing.55
Likewise, loncastuximab tesirine may be given as bridging therapy to CAR T cells. Despite being active toward CD19+ malignant lymphoid cells, treatment with loncastuximab tesirine typically does not cause loss of CD19 expression and therefore malignant cells should remain susceptible to effective CAR T cell–mediated antitumor activity. Indeed, a small case series of patients with DLBCL previously treated with loncastuximab tesirine reports a high probability to respond to CD19-directed CAR T-cell therapy (ORR 50%).41 However, as larger scale clinical data are lacking, an upcoming phase 2 clinical trial will address the appropriateness of its use in this scenario.
Summary
CD19-targeting ADCs represent a novel class of immunotherapy, which is highly effective, exhibits a rapid action, and can be safely administered as monotherapy in highly pretreated patients. In the future, they can be incorporated earlier in treatment course, both as monotherapy and in combination regimens, especially for patients who cannot tolerate standard therapy or require rapid debulking. Further, they may become a treatment option for certain subtypes of NHL with unfavorable biological characteristics and for patients failing cellular therapy and novel drugs.
As the armamentarium of drugs that exhibit significant activity against r/r DLBCL expands, the optimal choice of therapy in such complex and heterogenous disease is still a challenge. Numerous ongoing clinical trials will soon shed light on the therapeutic potential of CD19-targeting ADCs, as well as their optimal modality of use in the context of standard and novel treatment alternatives.
Acknowledgments
This work was supported in part by a grant from the Italian Association for Cancer Research (grant #20575 to C.C.-S.).
Authorship
Contribution: E.C., C.C.-S., and M.H. designed the review and all authors contributed to the writing and proofreading of the manuscript.
Conflict-of-interest disclosure: M.H. reports research support/funding from Takeda Pharmaceutical Company, ADC Therapeutics, Spectrum Pharmaceuticals, and Astellas Pharma; consultancy for Incyte Corporation, ADC Therapeutics, Pharmacyclics, Omeros, Verastem, TeneoBio, MorphoSys, Kite, Genmab, SeaGen, and Gamida Cell; speaker’s bureaus for Sanofi Genzyme, AstraZeneca, and BeiGene; P.L.Z. reports consultancy/advisory board member for Secura Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG therapeutics, Takeda Pharmaceutical Company, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte Corporation, and Beigene; speaker’s bureau for Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG therapeutics, Takeda Pharmaceutical Company, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte Corporation, and Beigene; P.C. reports research support/funding for Genentech, ADC Therapeutics; consultancy/advisory board member for Gilead, Genmab, Novartis, Genentech, Incyte Corporation, and Takeda; and speaker’s bureau for Bristol Myers Squibb. C.C.-S. reports research support/funding from ADC Therapeutics, Sanofi, and Roche; consultant/advisor for Celgene/Bristol-Myers Squibb, Genenta Science srl, ADC Therapeutics, Karyopharm Therapeutics, Roche, and Sanofi; and receives honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen Oncology, AstraZeneca, Incyte, Novartis, Takeda, and ADC Therapeutics. E.C. declare no competing financial interests.
Correspondence: Carmelo Carlo-Stella, Department of Oncology and Hematology, Humanitas Cancer Center, IRCCS, Via Manzoni 56, 20089 Milano, Italy; e-mail: carmelo.carlostella@hunimed.eu.

![Mechanism of action of PBD-ADCs (loncastuximab tesirine [Lonca]). (1) Lonca binds to CD19 on the tumor cell surface; (2) following internalization of Lonca, the protease-sensitive linker is cleaved and cytotoxic PBD dimers are released inside the cell; (3) the free PBD dimers bind in the minor groove of the cell DNA and form potent cytotoxic DNA cross-links in a sequence- selective fashion; (4) the cross-links result in a stalled DNA replication fork, blocking cell division; and (5) the cancer cell undergoes apoptosis.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/4/10.1182_blood.2021014663/3/m_bloodbld2021014663cf1.png?Expires=1769086475&Signature=Z4psKGTIPPEFu4os8I~DZ2drpqRWacbOjfNnGkiQR6wfuLLvvlybX4XQ6Ql8UsmrEbwAO7WHImmN5itGZRyRXUr-nDrj7HP72cvU2wWxwKRRsYgCGZ1GZAguBlhQFzY-e~HMAIFGI67ayAYDGidxMMFy4GsHTHqJcJeKebc7H~pjvOfXxlk3Galq~Rvhg8uuLhRdsbQm9nLk8Wr~Nqvy~SmyUohHhj1MWEADtPFMtq24I9QTVoqeGmGyLGXmm8~9KSYFeGrsLyP48VSf6GsU96hdzEolz20dcrr7AlYIs~l3nAXZ2n0VNR~VlZ0Sjk2PcgByN7gcui2euUeTHOMnOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal