Abstract
PTPN11 encodes for a tyrosine phosphatase implicated in the pathogenesis of hematologic malignancies such as Juvenile Myelomonocytic Leukemia (JMML), Acute Myeloid Leukemia (AML), and is associated with Acute Lymphoblastic Leukemia (ALL). Activating mutations of PTPN11 are known to increase proliferative signaling and cell survival, but the signaling upstream of PTPN11 is not well understood. Since PTPN11 is known to activate the MEK/ERK proliferative pathway there is significant interest in using MEK inhibitors such as trametinib for clinical benefit. Yet, as single agent, there appears to be minimal clinical activity. Previously, we showed that PTPN11 is further activated by an upstream tyrosine kinase TNK2, and that PTPN11-mutant JMML and AML cells are sensitive to TNK2 inhibition using dasatinib. Unfortunately, many cellular models include other gene mutations obscuring the exact mechanisms responsible for the proliferative effect and escape from MEK inhibition. In order to overcome this barrier we adapted a genetically engineered mouse model of PTPN11 driven leukemia. This model will prove to be extremely useful for preclinical validation of combination treatment for these diseases.
METHODS: We obtained the mouse strain 129S/Sv-Ptpn11tm6Bgn/Mmucd that carries a loxP-neo-loxP-STOP-loxP cassette between exons 2 and 3 of Ptpn11 and inserts a D61Y point mutation. These animals were then crossed with B6.129P2-Lyz2tm1(cre)Ifo/J that expresses the Cre recombinase under the control of the endogenous Lyz2 promoter/enhancer elements. The F1 progeny express Ptpn11D61Y mostly within hematopoietic cells destined along the granulocyte-monocyte progenitor lineage. Animals were monitored for disease by peripheral blood counts as well as behavior and well-being. At the time of sacrifice mononuclear cells were isolated from the bone marrow and spleen for subsequent in vitro assessments. MEK inhibition was performed using the small molecule inhibitor, trametinib. Single agent and combination treatment was also performed using the multi-kinase inhibitor, dasatinib as a surrogate for TNK2 inhibition. In vivo treatment was performed on cohorts of Ptpn11D61Y animals at approximately 7 months of age with daily drug treatment with dasatinib (40mg/kg/day), trametinib (1mg/kg/day) or a combination of dasatinib (40mg/kg/day) plus trametinib (1mg/kg/day) for 5 days each week for 3 months followed by assessment of disease burden.
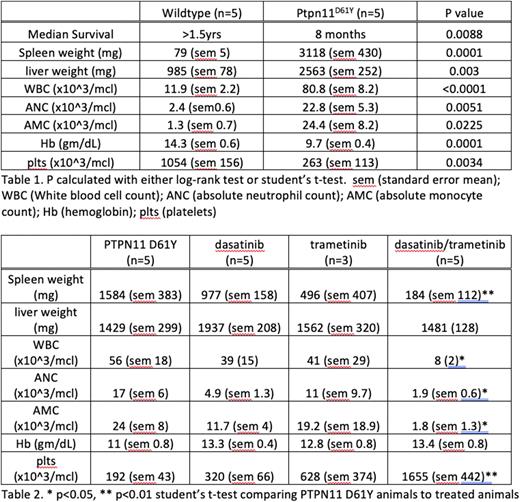
RESULTS: Consistent with the original characterization of the Ptpn11D61Y strain, the progeny expressing Ptpn11D61Y within hematopoietic cells developed a myeloproliferative disorder with a median survival of 8 months compared to their healthy littermates who survived for over 1.5 years to date (Table 1). Their disease was characterized by significantly enlarged spleen, liver, and a myeloproliferation with elevated total white blood cells, including neutrophils, and monocytes. The mutant animals also suffered from a consumptive anemia and thrombocytopenia.
Mononuclear cells extracted from the bone marrow or spleen of control animals showed minimal sensitivity to either dasatinib or trametinib (IC50 >1µM) compared to the Ptpn11D61Y cells (dasatinib, 50nM; trametinib, 5nM) in cell viability after exposure to drug for three days. Further, combination of dasatinib and trametinib showed chemical synergy (Bliss score of -5).
Cohorts of animals were then treated for 3 months with dasatinib, trametinib, or combination daily by oral gavage. At the end of three months there was a significant effect of the combination treatment in mitigating disease parameters compared to single agents (Table 2).
CONCLUSION: We have generated a simple mouse model of PTPN11 mutant driven myeloproliferative disorder. This model has validated the concept of combination therapy using dasatinib and trametinib for decreasing disease burden. Future studies will be performed to identify the molecular pathways affected by the combined use of these drugs within this model. Collectively, these data point to combined therapies as a promising target for PTPN11-mutant leukemias.
Disclosures
Tyner:Takeda: Research Funding; Syros: Research Funding; Seattle Genetics: Research Funding; Schrodinger: Research Funding; Petra: Research Funding; Meryx: Research Funding; Kronos: Research Funding; Janssen: Research Funding; Incyte: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Constellation: Research Funding; Astra-Zeneca: Research Funding; Array: Research Funding; Aptose: Research Funding; Agios: Research Funding; Acerta: Research Funding; Tolero: Research Funding; Recludix Pharma: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
preclinical use of dasatinib and trametinib for PTPN11 driven leukemias
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal