Abstract
Background Although pediatric regimens are useful treatment for acute lymphoblastic leukemia (ALL) in adolescents and young adults (AYA), the efficacy of risk stratification system used in pediatric regimens has hardly been investigated in AYA ALL. In addition, whereas nelarabine (NEL) is effective for patients with relapsed or refractory T-cell ALL (T-ALL), the role of NEL has not yet been well evaluated in the primary treatment of T-ALL. To assess the safety and efficacy of the pediatric regimen including NEL, intensified administration of L-asparaginase (L-ASP), and risk stratification by measurable residual disease (MRD) for T-ALL in AYA, the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG) and the Japan Adult Leukemia Study Group (JALSG) conducted JPLSG ALL-T11/JALSG T-ALL-211-U trial as an intergroup phase 2 study for patients with AYA with newly diagnosed T-ALL.
Methods The study was originally designed for patients aged 0-24 years. Herein, we report the results for patients aged 15-24 years. Patients were stratified into three groups based on the response to the prephase treatment with prednisolone(PSL), initial central nervous system (CNS) status, hematologic response after the induction therapy defined as time point one (TP1), MRD status using polymerase chain reaction after the early intensification therapy defined as time point two (TP2). Patients with good response to PSL (i.e., <1.0x109/L of blasts count in peripheral blood after prephase), no CNS involvement, complete remission (CR) at TP1, and MRD <10-3 at TP2 were assigned to the standard risk (SR) group. If patients had non-CR at TP 1 or MRD ≥10-3 at TP 2 regardless of PSL response and CNS status, they were assigned to the very high-risk (VHR) group. Patients who did not fulfill SR or VHR criteria were assigned to the high-risk (HR) group. If MRD evaluation at TP2 was not applicable, patients were deemed as having MRD <10-3. The treatment backbone was based on the Berlin-Frankfurt-Münster protocol, but intensification and maintenance with NEL (650mg/m2 x 5 days) were incorporated for HR and VHR groups. Further, according to the risk status, the total L-ASP dose was escalated to 210,000-420,000 U/m2. Applicating allogeneic hematopoietic stem cell transplantation (HSCT) was limited to patients in the VHR group.
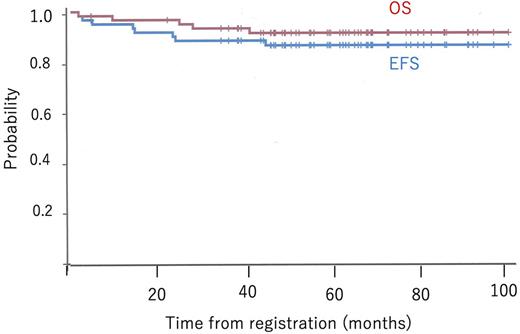
Results We analyzed 62 patients (21 females and 41 males) recruited between December 2011 and November 2017. The median patient's age and baseline absolute white blood cell counts were 17 years (range 15-24) and 32.2 x 109/L (range 0.8-506.3), respectively. CR or CR in suppression was achieved in 56 patients (90.3%) after the induction and early intensification therapies. Of these, MRD at TP2 was evaluable in 42 patients. Thirty-nine patients (70.0%) attained MRD <10-3 but MRD ≥10-3 was found in three (5.4%). Consequently, 26, 18, and 10 patients were categorized into SR, HR, and VHR groups. Eight patients dropped out of protocol before risk classification. Allogeneic HSCT in CR1was performed in nine patients in the VHR group. In total, the 3-year event-free survival (EFS) and overall survival (OS) were 88.6% (95% confident interval [CI]: 77.5-94.4) and 93.4% (95% CI: 83.4-97.5), respectively (Fig). The 3-year EFS in SR, HR and VHR groups was 96.2% (95% CI: 75.7-99.5), 88.9% (95% CI: 62.4-97.1), and 90.0% (95% CI: 47.3-98.5), respectively. The 3-year cumulative incidence of relapse was 5.3%. The most frequent non-hematologic grade ≥3 serious adverse event was hypofibrinogenemia (19%), followed by increased amylase (15%) and hypertriglyceridemia (8%). L-ASP dose was reduced in 19% of the patients due to adverse events. NEL-induced grade ≥3 neuropathy was seen in 7% of the patients who received NEL. One patient had non-relapse mortality because of gastric perforation during induction course.
Conclusions ALL-T11/T-ALL-211-U chemotherapy with NEL and intensified administration of L-ASP conferred excellent clinical outcomes for newly diagnosed T-ALL in AYA. Risk stratification system in pediatric regimen was also effective for AYA patients. The optimal dose of L-ASP should be further explored to minimize severe toxicities.
Disclosures
Hatta:Kyowa Kirin Co: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria; Novartis Pharma: Honoraria. Sato:Chugai: Honoraria. Miura:AstraZeneca: Honoraria; Chugai,: Honoraria; Kyowa Kirin: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Honoraria; Nippon Shinyaku: Honoraria; SymBio: Honoraria; Ono Parma: Honoraria. Kanda:asclepia: Honoraria; ASAHI KASEI PHARMA CORPORATION: Honoraria; Janssen Pharmaceutical K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb Co: Honoraria; Novartis Pharma K.K.: Honoraria, Membership on an entity's Board of Directors or advisory committees; TEIJIN PHARMA LIMITED.: Honoraria; CHUGAI PHARMACEUTICAL Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; DAIICHI SANKYO Co., Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; SymBio Pharmaceuticals, Ltd.: Membership on an entity's Board of Directors or advisory committees; Sanofi K.K.: Honoraria; Kyowa Kirin Co., Ltd.: Honoraria; Megakaryon Co: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutical K.K.: Honoraria; Ono Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Amgen Pharma Inc.: Honoraria; AbbVie Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Pharma Inc.: Consultancy, Honoraria; MSD K.K.: Honoraria; CSL Behring K.K.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; NIPPON KAYAKU CO.,LTD.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria; Eisai: Research Funding. Onishi:Meiji Seika: Honoraria; MSD: Honoraria; Astellas: Honoraria; Symbio: Honoraria; Kyowa Kirin: Honoraria; Amgen: Honoraria; BMS: Honoraria; Chugai: Honoraria; Abbvie: Honoraria; Takara Bio: Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Asada:Nippon Shinyaku: Speakers Bureau; Meiji: Speakers Bureau; Kyowa KIRIN: Speakers Bureau; Astellas: Speakers Bureau; Asahi KASEI: Speakers Bureau; Abbvie: Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Imai:Juno Therapeutics: Patents & Royalties; CURED Inc: Consultancy, Patents & Royalties, Research Funding. Horibe:Amgen Inc: Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Speakers Bureau; Novartis Japan: Speakers Bureau; Kyowa Kirin Co.,Ltd.: Consultancy; Pfizer Japan Inc.: Consultancy. Kiyoi:Meiji Seika Pharma Co., Ltd.: Honoraria; Amgen inc.: Honoraria; Bristol-Myers Squibb: Honoraria; SymBio Pharmaceuticals Limited: Honoraria; Novartis Pharma K.K.: Honoraria; AstraZeneca pic: Honoraria; Takeda Pharmaceutical Company Limited: Research Funding; JCR Pharmaceuticals Co.,Ltd.: Research Funding; Nippon Shinyaku Co.,Ltd.: Honoraria, Research Funding; AbbVie Inc.: Honoraria, Research Funding; Asahi Kasei Corporation: Research Funding; Astellas Pharma Inc.: Honoraria, Research Funding; CURED Co., Ltd: Research Funding; Perseus Proteomics Inc.: Research Funding; otsuka Pharmaceutical Co.,Ltd.: Research Funding; Daiichi Sankyo Co., Ltd: Honoraria, Research Funding; Eisai Co., Ltd.: Honoraria, Research Funding; Sumitomo Pharma Co., Ltd.: Research Funding; Zenyaku Kogyo Co., Ltd.: Research Funding; Kyowa Kirin Co., Ltd.: Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Pfizer Inc.: Honoraria; Nippon Kayaku Co.,Ltd.: Honoraria; Towa Pharmaceutical Co., Ltd.: Honoraria. Matsumura:Daiichi Sankyo Co., Ltd.: Speakers Bureau; Bristol-Myers Squibb K.K.: Speakers Bureau; Alexion Pharmaceuticals, Inc.: Research Funding; Pfizer Japan Inc.: Research Funding, Speakers Bureau; AbbVie G.K.: Research Funding, Speakers Bureau; SymBio Pharmaceuticals Ltd.: Speakers Bureau; Janssen Pharmaceutical K.K: Research Funding, Speakers Bureau; Astellas Pharma Inc.: Research Funding, Speakers Bureau; Novartis Pharma KK: Research Funding, Speakers Bureau; Mitsubishi Tanabe Pharma Corp.: Research Funding; Sanofi K.K.: Research Funding; Ono Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Nippon Shinyaku Co., Ltd.: Research Funding; Taiho Pharmaceutical Co., Ltd.: Research Funding; Eisai Co., Ltd: Research Funding; Asahi Kasei Pharma Corp.: Research Funding; Shionogi & Co., Ltd: Research Funding; Takeda Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Sumitomo Dainippon Pharma Co., Ltd.: Research Funding; Otsuka Pharmaceutical Co., Ltd.: Consultancy, Research Funding, Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Research Funding; Kyowa Kirin Co., Ltd: Research Funding. Miyazaki:Janssen Pharmaceutical: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; Dainippon-Sumitomo Pharma: Honoraria, Research Funding; SyinBio: Honoraria; Chugai: Honoraria; Bristol-Myers: Honoraria; Kyowa-Kirin: Honoraria; Pfizer: Honoraria; Otsuka Pharmaceutical: Honoraria; Takeda: Honoraria; Daiichi-Sankyo: Honoraria; Novartis: Honoraria; Nippon Shinyaku: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal