Abstract
Background: The thalassemias comprise a group of hemoglobinopathies characterized by ineffective erythropoiesis and hemolysis, which lead to chronic anemia and associated complications. Thalassemic red blood cells (RBCs) do not have sufficient levels of adenosine triphosphate (ATP) to meet their increased energy demands. Mitapivat is an allosteric activator of pyruvate kinase (PK), a key enzyme that regulates the final step of glycolysis responsible for ATP production in RBCs. We previously reported primary results of an open-label phase 2 study (NCT03692052) of mitapivat in pts with α- or β-non-transfusion-dependent thalassemia (NTDT) showing 80.0% (16/20) of pts achieved a hemoglobin (Hb) response of ≥1.0 g/dL increase from baseline at ≥1 assessments between Weeks (Wk) 4-12, inclusive. The increase in Hb was sustained, with a mean Hb (standard deviation) increase of 1.7 g/dL (0.5) at Wk 72; hemolysis markers were also improved. This analysis (data cutoff 27Mar22) evaluated the effect of mitapivat on markers of erythropoietic activity through Wk 72, along with Hb and markers of hemolysis and iron homeostasis.
Methods: Eligibility criteria: ≥18 years (y) of age, known medical history of α- or β-thalassemia, Hb concentration ≤10.0 g/dL, and ≤5 RBC units transfused in the prior 24 wk and none in the 8 wk prior to study drug. Pts started mitapivat 50 mg twice daily (BID) and escalated to 100 mg BID based on individual safety and Hb assessments. Pts who completed the 24-wk core period and achieved a Hb response or a delayed Hb response (after Wk 12) were eligible to continue to the extension period (at Wk 24 dose) if they had no ongoing grade ≥3 treatment-emergent adverse events related to study drug. Study visits during the extension occur every 12 wk for up to 3 y. Assessments collected within 8 wk (56 days) after a transfusion were excluded from baseline derivation and analysis.
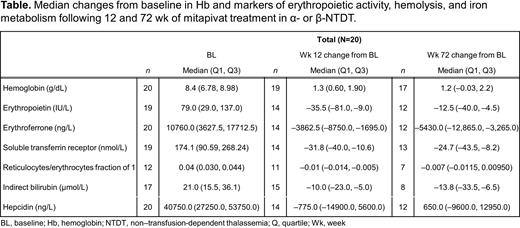
Results: Nineteen of 20 pts completed the core period, 17 entered the extension period; 16 pts (4 with α- and 12 with β-thalassemia) were ongoing at the data cutoff date. At baseline, pts had biomarker levels consistent with ineffective erythropoiesis and hemolysis (Table).
Changes from baseline showed a durable increase in Hb with a median (Q1, Q3) of 1.3 g/dL (0.60, 1.90) at Wk 12 and 1.2 g/dL (-0.03, 2.2) at Wk 72 (Table). Concurrently, there were trends for decreases in medians for erythropoietin, erythroferrone, soluble transferrin receptor, indirect bilirubin, and reticulocyte/erythrocyte fraction that were maintained over time. Median hepcidin levels remained relatively stable.
Conclusions: These results indicate that mitapivat's mechanism ameliorates multiple aspects of the complex pathophysiology underlying α- and β-NTDT, including ineffective erythropoiesis, hemolysis, and anemia. The sustained long-term effects of mitapivat may offer a novel disease-modifying approach.
Disclosures
Kuo:Celgene/BMS: Consultancy; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Honoraria; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy, Honoraria; Bioverativ/Sanofi/Sangamo: Membership on an entity's Board of Directors or advisory committees; Apellis: Consultancy; bluebird bio: Consultancy. Layton:Cerus: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lal:Agios: Research Funding; Bristol Myers Squibb: Research Funding; Forma Therapeutics: Research Funding; bluebird bio, Inc.: Research Funding; Celgene: Research Funding; Graphite Bio: Consultancy; La Jolla Pharmaceuticals: Research Funding; Novartis: Research Funding. Al-Samkari:Novartis: Consultancy; Amgen: Research Funding; Rigel: Consultancy; argenx: Consultancy; Forma: Consultancy; Moderna: Consultancy; Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Dova: Consultancy, Research Funding. Kosinski:Agios: Consultancy, Other: Shareholder. Tong:Agios: Current Employment, Current holder of stock options in a privately-held company. Estepp:Eli Lilly and Co: Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Emmaus Life Sciences: Consultancy; Daiichi Sankyo: Consultancy; Agios: Current Employment, Current equity holder in private company; Forma Therapeutics: Research Funding. Uhlig:Agios: Current Employment, Current holder of stock options in a privately-held company. Vichinsky:bluebird bio: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.
OffLabel Disclosure:
Mitapivat is a pyruvate kinase activator approved by the FDA for the treatment of hemolytic anemia in adults with pyruvate kinase (PK) deficiency. Mitapivat is not authorized for use by the EMA or any health authority outside the United States. The safety and efficacy of mitapivat in thalassemia is under investigation and has not been established. There is no guarantee that mitapivat will receive health authority approvals or become commercially available in any country for thalassemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal