Abstract
Background: Continuous inflammation and vasculopathy underlies the pathology of sickle cell disease (SCD). Therapeutics intervening the different stages in the pathophysiological cascade - polymerization of hemoglobin S (HbS) and "sickling" of the red blood cells (RBCs), vaso-occlusion and inflammation- are under development. Sickled RBCs initiate vaso-occlusion and have altered biomechanical properties that impact the rheology and hemodynamics in patients with SCD. There is an unmet need for robust, compact technologies that can non-invasively characterize the microvascular hemodynamics in SCD. Such a tool would be a valuable adjunct for clinicians both for broadening understanding of as well as monitoring therapeutic changes. However current clinical approaches can be time- and resource- intensive which limit their utility. Near-infrared spectroscopy (NIRS) is a non-invasive, optical technique for quantifying microvascular hemodynamics including hemoglobin concentration, tissue oxygen saturation, and blood flow (Cerussi et al, 2005). Here, we evaluate the sensitivity of NIRS to hemodynamic changes in patients with SCD undergoing treatment with Mitapivat (AG-348), a pyruvate kinase activator under clinical development to determine how well NIRS parameters compare with standard hematology markers.
Methods: We studied patients currently enrolled in a Phase I/II clinical trial (ClinicalTrials.gov, NCT04610866) that evaluates the long-term safety and tolerability of Mitapivat in the SCD population. We performed tissue hemodynamic assessments by affixing NIRS probes to the forearm and forehead of the subjects at baseline (prior to drug initiation) and over the course of three months of treatment with Mitapivat totaling five measurement visits per patient. The optical hemodynamic assessment consisted of resting baseline measurements followed by a brachial cuff occlusion and a recovery period. We acquired optical measures of hemodynamic markers, such as the concentration of oxy- and deoxy- hemoglobin and tissue oxygen saturation (StO2). Additionally, we calculated measures of vascular reactivity by analyzing the post-occlusion reactive hyperemic response. These metrics are compared for each patient across time to determine if there are any significant changes compared to baseline. Optical metrics were compared against changes in blood hemoglobin (Hb) level, an expected therapeutic effect of Mitapivat (Xu et al, 2022). Nine patients with complete NIRS data at baseline and spanning 3 months of Mitapivat were analyzed.
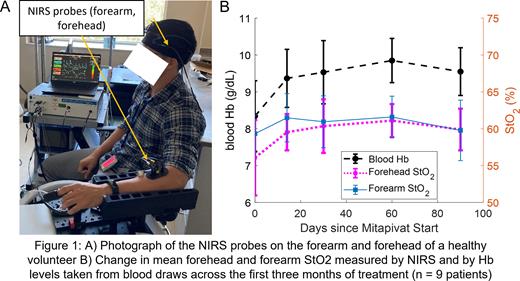
Results: We calculated optical hemodynamic metrics for all 45 measurement visits; one visit was excluded due to poor NIRS data quality. Figure 1A shows a typical measurement setup with NIRS probes acquiring simultaneous measurements from the forehead and forearm, which reflect tissue hemodynamics in the prefrontal cortex and skeletal muscle respectively. Figure 1B compares the mean Hb level, mean forehead StO2, and mean forearm StO2 across the first three months of treatment. On average, forehead StO2 acquired by NIRS trended similarly to the Hb level acquired by blood draws over the first three months. We observed that 7/9 patients exhibited a sustained increase in blood Hb compared to baseline; the mean Hb increase for all patients compared to baseline was 1.2 g/dL at the end of three months. Similarly, forehead StO2 measured by NIRS also showed a similar, sustained increase. The mean forehead StO2 increase after three months compared to baseline was 4.0%.
Discussion: We have demonstrated that NIRS could be a promising adjunct for non-invasively monitoring hemodynamics in patients with SCD undergoing treatment with Mitapivat. NIRS can provide objective measures of tissue composition and metabolism and vascular reactivity which can be performed in a simple, non-invasive measurement. Future analysis will include analyzing optical metrics of vascular reactivity and blood flow. While this initial cohort is small, the preliminary results are encouraging and warrant continued study into NIRS as a tool for monitoring hemodynamic changes promoted by other therapies for SCD.
Disclosures
Xu:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal