Abstract
INTRODUCTION: Acquired hemophilia A (AHA) is a potentially fatal hemorrhagic disease caused by the appearance of autoantibodies against factor VIII (FVIII). Immunosuppressive therapy (IST) is started immediately after diagnosis to eradicate FVIII inhibitors. This leads to remission in most patients, but infections often occur as side effects, leading to death in many cases. Emicizumab is a bispecific antibody that mimics the cofactor function of activated FVIII and prevents bleeds in patients with congenital hemophilia A regardless of inhibitor status. Emicizumab prophylaxis is expected to reduce the risk of severe bleeds in patients with AHA (PwAHA), and also allow at-risk patients to consider reducing or postponing IST, which would minimize its side effects. To investigate the safety and efficacy of emicizumab in PwAHA, we are conducting an ongoing, prospective, multicenter, open-label, non-randomized, phase III study (AGEHA; JapicCTI-205151). Primary analysis results of AGEHA suggest that emicizumab prophylaxis with a new loading regimen and completion criteria has a favorable benefit-risk profile in PwAHA. However, all enrolled patients in that primary analysis (cutoff date: April 23, 2021) had received IST before starting emicizumab, and the influence of IST, especially on efficacy data, cannot be completely ruled out. Here, we present efficacy and safety data of emicizumab in PwAHA deemed ineligible for IST at enrollment, which were collected after cutoff date of the primary analysis.
METHODS: AGEHA consisted of 2 cohorts: patients already undergoing or scheduled to immediately undergo IST at enrollment (Cohort 1), and patients deemed ineligible for IST at enrollment (Cohort 2). Emicizumab was administered subcutaneously at 6 mg/kg on Day 1 and 3 mg/kg on Day 2 followed by 1.5 mg/kg once weekly from Day 8. Predefined dosing completion criteria included FVIII activity >50 IU/dL. No restriction was set on IST use during the study. AGEHA was conducted in accordance with relevant ethical standards. Data from the 12 patients enrolled in Cohort 1 (Patients 1 to 12) is beyond the scope of this presentation.
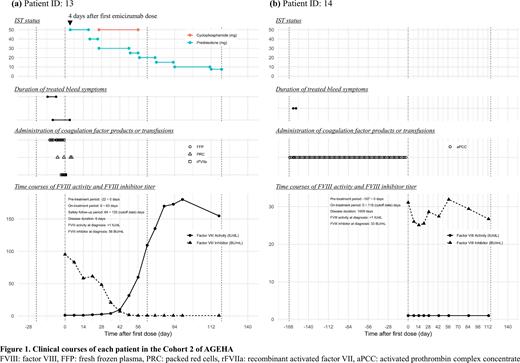
RESULTS: By the cutoff date for the presentation (September 10, 2021), 2 patients had been enrolled in Cohort 2, and 1 patient (Patient 13) had completed emicizumab treatment. Durations of emicizumab treatment were 7.1 weeks in Patient 13 and 16.3 weeks in Patient 14. Clinical courses of the 2 patients are shown in Figure 1.
Patient 13 was an 82-year-old female who was diagnosed with AHA 6 days before enrollment. She was judged ineligible for IST because of her poor performance status and diabetes. After starting emicizumab, the bleeding symptoms immediately disappeared and her general condition dramatically improved. Following treatment with intensive insulin therapy for diabetes, the investigator judged that IST was applicable to her. IST with prednisolone and cyclophosphamide was started on Day 5 and Day 27, respectively, and then FVIII inhibitor titer decreased and FVIII activity gradually improved. Criteria for completion of emicizumab were met on Day 64.
Patient 14 was a 59-year-old female with AHA of long disease duration (1009 days) who had been receiving a prophylactic regimen of activated prothrombin complex concentrate before enrollment. She was judged ineligible for IST because IST had not been effective for a long time in the past, and because IST was nearly contraindicated due to a complication of infections associated with osteonecrosis of the jaw.
During the pre-treatment period, the 2 patients experienced a total of 3 treated bleeds, 1 of which was a treated major bleed in Patient 13. During the on-treatment period, no treated bleeds and no major bleeds occurred in either patient. The requirement for coagulation factor products or transfusions decreased between the pre- and on-treatment periods. Both patients experienced adverse events (AEs), all 9 of which were grade 1 or 2. One AE (rash) in Patient 13 was considered related to emicizumab, but no modification or discontinuation of emicizumab was required. No thrombotic events, serious AEs, AEs leading to study discontinuation, or anti-drug antibodies were reported.
CONCLUSIONS: These results suggest that emicizumab prophylaxis with the new dosing regimen and completion criteria is safe and efficacious for PwAHA ineligible for IST. Further, efficacy under the non-influence of IST was also suggested.
Disclosures
Suzuki:Chugai Pharmaceutical Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Sanofi K.K.: Honoraria; Novo Nordisk Pharma Ltd.: Honoraria; Bayer Yakuhin, Ltd.: Honoraria; Pfizer Inc.: Honoraria; CSL Behring LLC: Honoraria; KM Biologics Co., Ltd.: Honoraria; Japan Blood Products Organization: Honoraria. Kobayashi:Chugai Pharmaceutical Co., Ltd.: Current Employment. Mizuno:Chugai Pharmaceutical Co., Ltd.: Current Employment, Current equity holder in publicly-traded company. Ozaki:Chugai Pharmaceutical Co., Ltd.: Current Employment. Shima:Takeda: Honoraria, Research Funding; UniQure: Consultancy; Sanofi: Consultancy, Honoraria, Speakers Bureau; Fujimoto Seiyaku: Consultancy, Honoraria, Speakers Bureau; Bayer Yakuhin: Honoraria; F. Hoffmann-La Roche Ltd.: Speakers Bureau; Chugai Pharmaceutical Co., Ltd.: Consultancy, Honoraria, Research Funding, Speakers Bureau; Nara Medical University: Current Employment; Ono Yakuhin: Speakers Bureau; Novo Nordisk: Honoraria, Speakers Bureau; CSL Behring: Honoraria, Research Funding, Speakers Bureau.
OffLabel Disclosure:
Emicizumab for acquired hemophilia A: Approved in Japan but not in other countries including the United States of America
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal