Abstract
Introduction: Venetoclax (ven) with azacitidine (aza) is the standard of care for newly diagnosed acute myeloid leukemia (AML) patients (pts) who are not candidates for intensive chemotherapy (IC). Efforts are needed to further optimize this regimen. In a previous report, measurable residual disease negativity (MRD-) was achieved in 41% of responders; these pts had superior outcomes. We hypothesized ven doses >400mg could be safely administered and would produce deeper remissions. In addition, responding pts continue treatment indefinitely. Given the prognostic significance of MRD-, we hypothesized MRD would identify candidates who could de-intensity therapy. To test these hypotheses, we designed an investigator initiated study in which newly diagnosed older AML pts were given 600mg of ven with aza. Those who achieved MRD- discontinued aza and were maintained on ven alone (High Dose Discontinuation Aza+Ven Study: HiDDAV).
Methods: Pts were eligible if they had no prior therapy, were ≥60, ineligible for IC, had adequate organ function and a white blood cell count <25x109/L. Pts were admitted for "induction": dose escalation of ven to 600 mg over a 4 day period (100, 200, 400, 600), continued until day 28, and concomitant aza 75 mg/m2 on d 1-7. Pts without a CR/CRi/MLFS by day 28 either discontinued or repeated an induction cycle, as did those who achieved CR/CRi/MLFS but were MRD+. Up to 3 inductions were permitted for non-responders or MRD+ responders. If pts achieved a response without MRD- after the 3rd induction cycle they moved to MRD+ maintenance: ven 400 mg d 1-28 with aza d 1-7. From the conclusion of the first induction, and continuing with each response assessment, any pt who achieved CR/CRi/MLFS with MRD- went to MRD- maintenance: ven 400 mg d 1-28 and discontinuation of aza. Two MRD assessments were employed: multiparameter flow cytometry (flow) and droplet digital polymerase chain reaction (ddPCR) assays for as many baseline somatic non-DNMT3A/TET2/ASXL1 (DTA) mutations as possible. Full MRD- was defined as MRD- by flow and ddPCR.
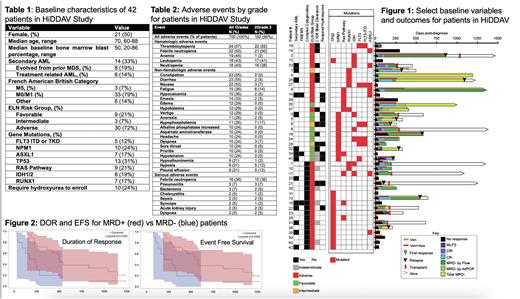
Results: Baseline data for the 42 pts is summarized in Table 1. Median follow up time is 42 months. 32 pts (76%) had a serious AE (SAE). Common AEs (≥20%) are summarized in Table 2. The most common hematologic AEs ≥ Grade 3 included thrombocytopenia (52%), febrile neutropenia (50%), leukopenia (41%) and neutropenia (38%). 30 day mortality occurred in 5/42 (11.9%), all of whom had refractory disease.
The overall response rate was 28/42 (66.7%); 26 (61.9%) had a CR and 2 (4.8%) had MLFS. GCSF was used in 17/28 responders. Of 28 responders, 18 (64.3%) were MRD- by flow. 26/28 (93%) were able to be monitored by ddPCR; the percentage of all baseline non-DTA genes able to be monitored by ddPCR was 70% (45/64). Of pts monitored by ddPCR, 9/26 (35%) achieved MRD-. Full MRD- was achieved in 7/28 (25%). 10 went to transplant. Individual outcomes are summarized in Figure 1.
The median duration of response (DOR) was 12.9 months (m) (95% CI=7.4, 17.6), event free survival (EFS) was 7.8 m (95%CI=2.5, 11.2) and OS was 9.8 m (95% CI=6.6, 14.9). MRD- by ddPCR, and full MRD-, did not associate with OS, EFS or DOR. However, MRD- by flow had significantly improved EFS (median 18.7 vs 8.2 m, p=0.047) and DOR (median 16.6 vs 6.7 m, p= 0.037), and a longer OS (19.6 vs 11.2 m, p=0.16) (Figure 2).
Aza was discontinued in 12 pts; in 8 this was protocol mandated due to full MRD-, and occurred after a median of 2 cycles. 4 pts chose to stop aza, in violation of the protocol without MRD-, after a median of 4 cycles. The median DOR, EFS and OS for those who stopped aza with and without guidance was 13.9 m (95% CI=7.1, NR) and 12.9 m (95% CI=5.5, NR), 15.4 m (95% CI=8.5, NR) and 14.3 m (95%CI=6.6, NR), and 15.4 m (95% CI=8.5, NR) and 18.8 m (95% CI=6.6, NR), respectively.
Conclusions: Higher rates of MRD- were seen with 600mg ven than has been reported with 400mg, and MRD- by flow was associated with improved clinical outcomes. However, DOR, EFS and OS were not improved over what has been reported with ven 400mg. Monitoring MRD by ddPCR was feasible, but in this dataset, this more stringent method of MRD detection did not identify pts with better outcomes. Pts whose aza discontinuation was guided by MRD- did not have improved outcomes compared to pts who discontinued aza without such guidance. Effective methods to de-intensify therapy in appropriate pts remain necessary.
Disclosures
McMahon:Syros: Research Funding; Arcellx: Consultancy. Smith:Syros: Research Funding; Kura: Research Funding; AML JV: Research Funding; Argenx: Research Funding.
OffLabel Disclosure:
Higher doses of venetoclax than labeled
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal