Abstract
The three constitutively active PIM serine/threonine kinases have been previously reported to initiate and sustain lymphoid oncogenesis. In subsequent CRISPR/Cas9 drop-out screens, PIM kinases were highlighted as critical oncogenes in MM, which led to clinical trials of small-molecule PIM kinase inhibitors. Surprisingly, PIM inhibitors in MM patients exhibited limited activity due to their cytostatic, rather cytotoxic effects. These clinical trial results prompted us to further investigate the consequences of PIM kinase inhibition in MM using recently developed pan-PIM/FLT3 inhibitor MEN1703.
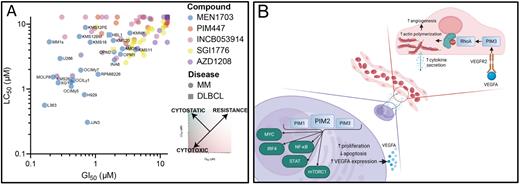
Immunohistochemical staining revealed a markedly increased PIM1/2/3 levels in malignant plasma cells in comparison with normal plasma cells from healthy donors, with particularly abundant PIM2 expression. Through the integration of CRISPR/Cas9 dropout screen and genome-wide H3K27Ac profiling in MM cells, we identified PIM2 as a super-enhancer-driven oncogene in MM. BET inhibitor JQ1, which disrupts super-enhancer assembly, downregulated PIM kinase expression. Since super-enhancer associated genes are often the most essential nodes of key cellular pathways to be potentially targeted, these results further underscore the critical role of PIMs in MM. Next, we compared the activity of MEN1703 with all available clinical-stage PIM inhibitors in a panel of >20 different cell lines and found almost universal resistance to 4 out of 5 pan-PIM inhibitors administered at submicromolar concentrations achievable in vivo (Fig. 1A). In contrast, MEN1703 exhibited universal cytotoxic activity. We confirmed these results using patient-derived multiple myeloma cells cultured ex vivo and genetic knockdowns of PIM kinases. Mechanistically, MEN1703 decreased the activity of mTOR, JAK/STAT, and NFκB pathways essential for supporting myeloma cell survival. Consequently, we observed diminished expression of MYC and IRF4, two transcription factors crucial for supporting plasma cell-specific oncogenic program.
In the IHC analysis of PIMs expression in MM tissue microarray, we also noted a distinctly high expression of PIM3 in myeloma-associated endothelial cells (ECs), but not in healthy bone marrow ECs. Since ECs play a significant role in shaping the bone marrow microenvironment and directly support the dissemination and survival of plasma cells, we studied the mechanism of PIM3 overexpression, PIM3 function, and the consequences of its inhibition in MM ECs. We found that myeloma-secreted VEGFA is responsible for PIM induction in ECs and that endothelial cells reciprocally induce PIMs expression in MM cells in a paracrine manner, thus increasing their proliferation and inhibiting cell death. PIM inhibition blocked these effects, which was confirmed using cocultures with primary MM endothelial cells. PIM inhibition decreased the MM EC vessel formation by decreasing the activity of endothelial GTPase RhoA, which is responsible for actin cytoskeleton formation during angiogenesis and cytokine secretion.
Collectively, our data demonstrate that PIM inhibition with MEN1703 induces MM cell death and abolishes important tumor cell-ECs interactions (Fig. 1B), thus representing a promising therapeutic target in this disease. The clinical utility of next generation/more potent PIM inhibitor in MM should be re-investigated.
Study supported by National Science Centre Grants PRELUDIUM 2018/31/N/NZ5/03214, ETIUDA 2020/36/T/NZ5/00610 and Ministry of Science and Higher Education Diamond Grant DI2015 007145.
Disclosures
Brzózka:Selvita SA: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Nodthera: Current equity holder in publicly-traded company; Ardigen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Rzymski:Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Bellarosa:Menarini Group: Current Employment. Sewastianik:Affini-T Therapeutics: Current Employment. Juszczynski:RYVU Therapeutics: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal