Abstract
Introduction: Studies of multiple myeloma (MM) populations indicate that patients with more advanced and heavily pretreated disease have worse health-related quality of life (HRQoL) than those at earlier stages. In addition, treatment options are limited for triple-class exposed relapsed/refractory MM (RRMM), with little hope of improving HRQoL. Talquetamab is a first-in-class, off-the-shelf, T-cell redirecting bispecific antibody targeting both GPRC5D and CD3 receptors. MonumenTAL-1 is a phase 1/2 trial (NCT03399799/NCT04634552) of talquetamab in patients with RRMM. Patient-reported outcomes (PROs) data on HRQoL, symptoms, and functioning were collected in the phase 2 cohorts of MonumenTAL-1. Here, we report PROs, focusing on global health status (GHS), physical functioning, pain, and fatigue, for the 0.4 mg/kg subcutaneous (SC) weekly (QW) cohort. Data for the 0.8 mg/kg SC every 2 weeks (Q2W) cohort are immature currently.
Methods. Patients enrolled in phase 2 must have previously received ≥3 prior lines of therapy, including ≥1 proteosome inhibitor, ≥1 immunomodulatory drug, and ≥1 anti-CD38 monoclonal antibody (ie, triple-class exposed). After screening, patients received talquetamab step-up doses, requiring hospitalization, before cycle 1. The European Organization for Research and Treatment of Cancer Quality of Life Core 30-item (EORTC QLQ-C30) questionnaire is a cancer-specific PRO instrument administered at screening, cycle 1, and then every other cycle. It includes a subscale to measure HRQoL, 5 functional scales, 3 symptom subscales, and 6 additional single items. Scores range from 0 to 100; higher scores indicate better GHS and functioning, whereas a higher score represents more symptom severity. Compliance with PROs was calculated as the number of completed assessments divided by the number of patients on study treatment at each assessment time point. Treatment effect was assessed with a mixed-effects model with repeated measures. For the analysis of the proportion of patients with meaningful improvement, the threshold for meaningful improvement from baseline was defined as a change of ≥10 points. Time to worsening was determined using the Kaplan-Meier estimate.
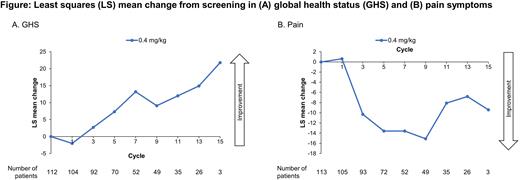
Results: For patients treated with 0.4 mg/kg SC QW (n=122), compliance with completing the EORTC QLQ-C30 was 96% at screening and over 80% at most post-treatment visits. After an immediate decline in overall HRQoL between screening and cycle 1, patients reported meaningful improvements in EORTC QLQ-C30 GHS and a meaningful reduction in pain symptoms compared with baseline (Figure). Least squares [LS] mean change for fatigue symptoms was -8 (95% CI, -13.83, -2.26) at cycle 9 and reached a mean decrease (ie, improvement) of 11.2 points (95% CI, -18.9, -3.53) at cycle 13. Similar results were observed in the physical (LS mean change at cycle 9: 6.5 [95% CI, 2.05, 10.93]) and role (LS mean change at cycle 9: 11.4 [95% CI, 4.55, 18.25]) functioning subscales. With treatment, the proportion of patients with meaningful improvement was high; for example, at cycle 9 for the 0.4 mg/kg cohort, 42% improved in GHS, 34% in physical functioning, 40% in role functioning, 86% in pain symptoms, and 78% in fatigue symptoms. Median time to worsening ranged from 2 (role and social functioning) to 9 months (nausea/vomiting). Overall, changes in PROs were similar in the 0.8 mg/kg SC Q2W cohort in early cycles, but conclusions are limited due to short follow-up and small sample size. The 0.8 mg/kg SC Q2W cohort will be updated with additional follow-up.
Conclusions: With talquetamab treatment, patients in the 0.4 mg/kg SC QW cohort reported improvement in overall HRQoL and physical and role functioning and a decrease in pain and fatigue. These findings are consistent with the clinical benefits of talquetamab as demonstrated by the efficacy results from the MonumenTAL-1 study.
Disclosures
Schinke:Janssen: Honoraria. Minnema:Bristol Myers Squibb: Speakers Bureau; Medscape: Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Berdeja:Lilly: Research Funding; Genentech: Research Funding; Ichnos Sciences: Research Funding; AbbVie: Research Funding; Acetylon: Research Funding; CARsgen: Research Funding; Janssen: Consultancy, Research Funding; Kite Pharma: Consultancy; Takeda: Consultancy, Research Funding; Legend Biotech: Consultancy; SecuraBio: Consultancy; Amgen: Research Funding; C4 Therapeutics: Research Funding; Incyte: Research Funding; Novartis: Research Funding; Karyopharm: Research Funding; 2Seventy bio: Research Funding; Cartesian Therapeutics: Research Funding; Celularity: Research Funding; Zentalis: Research Funding; Fate Therapeutics: Research Funding; GlaxoSmithKline: Research Funding; Poseida: Research Funding; Teva: Research Funding; EMD Sorono: Research Funding; Sanofi: Consultancy, Research Funding; Bluebird bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding. Oriol:Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy; GlaxoSmithKline: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Van De Donk:Cellectis: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding. Rodriguez Otero:Janssen, BMS, Sanofi, Pfizer, GSK.: Consultancy; Amgen, Sanofi, GSK, Janssen, BMS-Celgene, Regeneron: Speakers Bureau; Regeneron Pharmaceuticals, Inc.: Speakers Bureau; BMS-Celgene: Speakers Bureau; Amgen: Speakers Bureau; GSK: Consultancy, Speakers Bureau; Pfizer: Consultancy; Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; BMS: Consultancy; Hematology Clínica Universidad de Navarra: Current Employment. Mateos:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Costa:AbbVie: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Genentech: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Caers:Amgen: Speakers Bureau; Johnson & Johnson: Consultancy, Research Funding; Sanofi: Honoraria. Rasche:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Krishnan:Takeda, GSK, BMS: Speakers Bureau; Janssen: Research Funding; BMS: Current equity holder in publicly-traded company; BMS, Janssen, Adaptive, GSK, AbbVie, Regeneron, Sanofi, AstraZeneca: Consultancy; Artiva: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy, Research Funding; Regeneron: Consultancy; Adaptive: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Sutro: Consultancy; Bristol Myers Squibb: Consultancy, Other: Stock Ownership (not including stocks owned in a managed portfolio), Speakers Bureau; GlaxoSmithKline: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Sutro SAB: Speakers Bureau. Ma:Janssen: Current Employment. Qin:Janssen: Current Employment. Gries:Janssen Pharmaceutical: Current Employment, Current holder of stock options in a privately-held company. Kato:Janssen: Current Employment. Campagna:Janssen: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Masterson:Janssen R&D, LLC: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hilder:Janssen: Current Employment. Tolbert:Janssen Pharmaceuticals: Current Employment. Renaud:Janssen: Current Employment. Goldberg:Janssen: Current Employment, Current equity holder in private company. Heuck:Janssen R&D: Current Employment, Current equity holder in publicly-traded company. Moreau:AbbVie, Janssen, Celgene, Amgen, and Sanofi: Honoraria. San-Miguel:Abbvie, Amgen, BMS, Celgene, GSK, Haemalogix, Janssen-Cilag, Karyopharm, MSD, Novartis, Takeda, Regeneron, Roche, Sanofi, and SecuraBio: Consultancy, Other: Advisory Board.
OffLabel Disclosure:
at the time of abstract submission, talquetamab is being investigated for the treatment of multiple myeloma but is not yet not approved
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal