Abstract
Introduction: Allogeneic haematopoietic stem cell transplant (alloHCT) is a potentially curative therapy for many serious haematological disorders. The advent of alternative donor alloHCT, such as umbilical cord blood unit (CBU) and haploidentical (HAPLO), greatly widened the donor pool for recipients without HLA (human leukocyte antigen)-matched donor (MD), providing near universal donor availability. Although survival outcome of MD and alternate donor is considered to be similar, there is ongoing debate about preference of CBU and HAPLO (Wagner et al Blood Adv 2021; Wieduwilt et al Blood Adv 2022). Increased relapse after HAPLO versus increased non-relapse mortality with CBU makes selection of alternative donors difficult, with minimal supporting evidence. Hence, we compared single-centre outcomes of MD versus alternative donor alloHCT.
Method: We performed a retrospective, consecutive cohort review of all patients who underwent alloHCT between 1st Jan 2010 and 30th Jun 2021 at our centre. Donors matched with recipients at HLA-A, HLA-B, HLA-C and HLA-DRB1, HLA-DQB1 loci (10/10) were considered MD, HAPLO (5/10) and CBU matched (5 - 6/8).
Graft versus Host Disease (GVHD) prophylaxis for MD and CBU was calcineurin inhibitor, whereas HAPLO utilised post-HCT cyclophosphamide. Unrelated MD recipients also received rabbit anti-thymoglobulin (rATG) for in vivo T cell depletion.
Neutrophil engraftment was defined as first of three consecutive days of absolute neutrophil count (ANC) > 0.5x109/L. Platelet (PLT) engraftment was defined as first of three consecutive days of PLT count > 20x109/L, unsupported for ≥7 days.
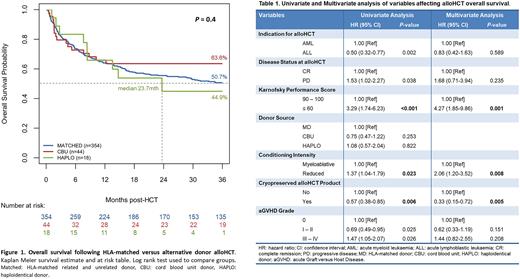
Overall survival (OS) was assessed by Kaplan Meier (KM) method. Cumulative incidence (CI) of GVHD and relapse (REL) were estimated as competing events. Univariate and multivariate analysis (U/MVA) was conducted via Cox regression model. Statistical significance was defined as P<0.05.
Results: Four-hundred and sixteen alloHCT were performed at our centre: 354 (85.10%) MD (41% related and 59% unrelated), 44 (10.58%) CBU and 18 (4.33%) HAPLO. Median follow up was 65.12 months. CBU recipients were significantly younger than other MD or HAPLO (median age 52 vs 45 vs 61 years, respectively: P=0.0001). Primary indication for transplant of total cohort was myeloid malignancy (64%) with majority in complete remission (68%) at transplant - there was no significant difference between donor groups. MD alloHCT utilised myeloablative conditioning significantly more than CBU and HAPLO (57% vs 23% vs 11%, respectively: P<0.0001). Median CD34 dose infused was 5.02 (4.28 - 5.98), 0.29 (0.24 - 0.36) and 4.98 (4.37 - 5.00) x106/kg for MD, CBU and HAPLO, respectively (P=<0.0001).
There was no difference in ANC recovery (median D+19 vs 19 vs 25 for MD, CBU and HAPLO, respectively: P=0.2) between donor groups. PLT recovery was significantly faster for MD than CBU and HAPLO (median D+23 vs 41 vs 38, respectively: P=0.0139).
Cumulative incidence of Grade II - IV acute GVHD (aGVHD) by D+100 was significantly higher in CBU than MD (48.46% vs 23.06%: P<0.001) and HAPLO (48.46% vs 34.81%: P=0.001). There was no difference of aGVHD CI between MD and HAPLO (P=0.2).
Cumulative incidence of relapse was significantly higher following HAPLO versus CBU (3yr CI 52% vs 23%: P=0.025). However, there was no difference in REL between MD and CBU (32% vs. 23%; P=0.1) or MD and HAPLO (32% vs. 52%; P=0.3).
Median and 3-year OS in our total cohort was 40.1 months and 51.8% (95% CI 0.570), respectively. We did not observe OS difference according to donor groups. Three-year OS was 51%, 63% and 45% for MD, CBU and HAPLO, respectively (P=0.4). UVA did not identify OS difference between donor groups. However, UVA revealed significant difference in other variables that were reassessed by MVA, per Table 1. Interestingly, MVA demonstrated significantly lower OS in fresh versus cryopreserved (cryo) alloHCT (HR fresh vs cryo 0.33, 0.15 - 0.72, P=0.005).
Conclusion: Overall, we observed higher rates of aGVHD with CBU, versus higher rates of REL for HAPLO. However, the overall survival was comparable. Our results demonstrate that with careful patient selection and optimal management, alternative donor alloHCT have comparable outcomes to MD.
Disclosures
Sharplin:Kite: Honoraria; Novartis: Other: Travel and conference funding. Shanmuganathan:Novartis: Honoraria; Amgen: Other: Meeting sponsorship. Yeung:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Honoraria, Research Funding; Pfizer: Honoraria; Amgen: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Horvath:bms: Research Funding; sanofi: Research Funding. Hiwase:BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; AbbVie: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal