Abstract
Introduction: The metalloprotease ADAMTS13 consists of metalloprotease (M), disintegrin-like (D), thrombospondin-1 (T), Cys-rich (C), and spacer (S) domains (proximal domains), followed by 7 T and 2 CUB domains (distal domains). ADAMTS13 cleaves the cryptic Tyr1605-Met1606 bond in the A2 domain of von Willebrand factor (VWF) to prevent ultra-large multimeric VWF-induced platelet aggregation. We and others previously described the allosteric regulation of ADAMTS13 by which the distal domains fold back and inhibit the proximal domains. VWF D4-CK domains bind the ADAMTS13 T8-CUB region to disrupt this allostery and accelerate VWF proteolysis. We recently discovered that ADAMTS13 and VWF have not evolved to be optimal enzyme-substrate pairs (Muia, Blood, 2019). For example, c-VWF71 (C-71), a wild-type bovine VWF domain A2-derived peptide substrate, is cleaved extremely well by many vertebrate ADAMTS13. Conversely, similar substrates prepared from wild-type human h-VWF71 (H-71) and other VWF domain A2 sequences mostly tolerated cleavage by their ADAMTS13 or additional few species-specific ADAMTS13. Although unfolded VWF A2 domain engages ADAMTS13 M, D, C, and S exosites for substrate specificity and efficient cleavage, sequence differences among the various vertebrates VWF71 substrates may hold clues about specificity determinants of ADAMTS13 substrate recognition and cleavage.
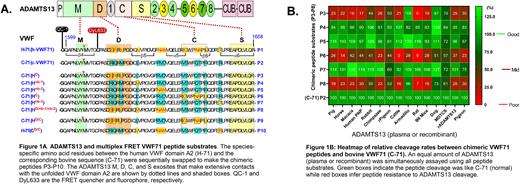
Methods: Multiplex FRET ADAMTS13 assays were performed using 10 novel fluorogenic substrates. Substrates P3-P10 are chimeric substrates between C-71 and H-71; species-specific amino acid residues were swapped sequentially (Fig 1A). Plasmas from various vertebrates have been characterized and reported before. Recombinant ADAMTS13 variants were transiently expressed in HEK293 cells. Assays were performed as previously described and mean results are reported.
Results: The chimeric substrates (P3-P10) were designed to substitute wild-type, species-specific amino acid residues at sites known to interact with ADAMTS13 D and C domain exosites. However, amino acid substitutions also occurred at the sites that do not interact directly with any ADAMTS13 exosite. Compared to C-71 (P2), only P3 and P6 exhibited reduced rates of cleavage by all vertebrate plasma containing ADAMTS13 (Fig 1B). The P3 substrate substituted the D binding residues from human VWF71 into C-71, whereas P6 substituted the C binding residues from human VWF71 into C-71. For P3, all ADAMTS13 assayed showed ~30-100% reduction in the rate of cleavage except for armadillo and dog plasma ADAMTS13. Likewise, P6 was cleaved ~0% to 60% slowly relative to P2 by all ADAMTS13. Substituting the D, nb-1(non-binding region 1), and nb-2 sequences from human into C-71, named P8, did not further reduce rates of cleavage compared to P3. These results suggest the nb-1 and nb-2 regions of VWF71 do not participate in protease engagement, consistent with previous studies. We wondered if swapping human VWF D- and C- binding sites to C-71 as well as the bovine's to H-71 would result in ADAMTS13 cleavage rates similar to wild-type sequences. Interestingly, there was no major change in cleavage rates when D- and C- VWF binding sites were of the same species, i.e., P9 ~ P1 and P10 ~ P2, irrespective of the peptide backbone. We also sought to know if allosteric regulation of ADAMTS13 attenuates the binding of these chimeric substrates to ADAMTS13. Pigeon (potently activated by low pH) and human plasma ADAMTS13 were activated ~15.0-28.0 and ~4.8-8.0-fold, respectively when assayed with all 10 peptides. Whether ADAMTS13 was activated or not, the ratio of cleavage for individual substrates to the parental P2 remained relatively constant (~ 0.4-1.3), implying the substrate-dependent activity is independent of allosteric activation of ADAMTS13.
Conclusions: The fact that D- and C- VWF binding sites from human and bovine VWF71 cannot be combined without negatively affecting the Tyr-Met bond cleavage may suggest coupling of ADAMTS13 D and C domains to regulate its activity. It is plausible to claim that ADAMTS13 VWF D- and C- binding sites and/or ADAMTS13 D and C domains may have evolved to provide a fail-safe point for allosterically activated ADAMTS13. This may prevent substrate-activated ADAMTS13, which precedes the unraveling of the VWF domain A2, from engaging and cleaving off-targets. We conclude that VWF as a substrate serves both as an ADAMTS13 activator and activity regulator.
Disclosures
Vanhoorelbeke:Takeda: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal