Abstract
CD19 directed chimeric antigen receptor T cell (CART19) therapy has led to an unprecedented success in B cell malignancies. However, the majority of patients relapse, and rates of durable remissions are low. The predominant mechanism of CART cell failure is the inhibition of CART cell activation by the tumor microenvironment. It has become apparent from preclinical studies and correlative science that CART cells rely on death receptors and pathways such as FasL:Fas and TRAIL: TRAIL receptors to exert their full effector functions.
In this study, we aimed to investigate the impact of TRAILshort on CART cell functions. TRAILshort is a splice variant that functions as a dominant negative ligand for full length TRAIL binding to apoptosis inducing TRAIL receptors DR4 and DR5, thereby impairing immune effector cell functions. TRAILshort is expressed at high levels in the settings of chronic viral infections and in cancer. We therefore hypothesized that targeting TRAILshort may improve CART cell functions. To test our hypothesis, we used TRAILshort targeting monoclonal antibodies (mAbs) and studied how they impact CART19 effector functions in models for B cell malignancies. In addition, we tested targeting TRAILshort expressing cells with TRAILshort directed CART cells. Finally, we tested dual targeting of CD19 and TRAILshort (CD19/TRAILshort CART cells).
First, we confirmed high level of TRAILshort expression in B cell malignancies, including primary B-acute lymphoblastic leukemia, primary mantle cell lymphoma, the mantle cell lymphoma JeKo-1 cell line, as well as in control Jurkat, and HeLa cells, both by qPCR and flow cytometer. Next, we studied the effect of TRAILshort neutralization using a humanized TRAILshort antibody (α-huTRAILshort) on CART19 effector functions. A co-culture of CART19 with the CD19+ cell line JeKo-1 in the presence of α-huTRAILshort antibody demonstrated increased CART19 proliferation (p= 0.0029) and cytotoxicity (p= 0.0055), compared to the matched isotype control. We then verified the in vivo antitumor activity of CART19 cells in the presence of α-huTRAILshort in a JeKo-1 xenograft model. NOD-SCID-γ-/- (NSG) mice were engrafted with the luciferase+ CD19+ JeKo-1. Two weeks later, engraftment was confirmed by bioluminescent imaging and mice were randomized to treatment with CART19 (1x106 cells intravenously) in combination with α-huTRAILshort monoclonal antibody or isotype control (10 mg/Kg intraperitoneally). The combination of CART19 and anti huTRAILshort resulted in improved tumor control compared to controls (p=0.0357).
Next, we targeted TRAILshort directly with TRAILshort specific CART cells. We therefore generated TRAILshort directed CART cells (CARTS1) using lentiviral transduction of normal donor T cells with a TRAILshort CAR vector encoding 41BB and CD3ζ signaling domains.
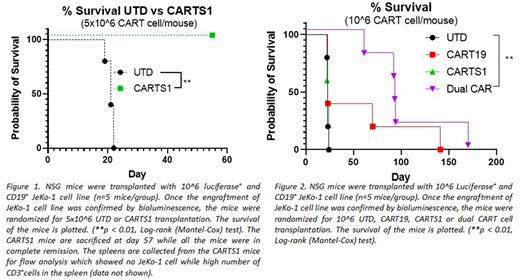
As compared to control untransduced T cells (UTD), CARTS1 cells exhibited potent antigen specific killing of HeLa (p <0.0001), Jurkat (p= 0.0158) and JeKo-1 (p= 0.0017) cell lines, but not of the TRAILshort negative cell line TRAILk/o HeLa. To rule out T cell fratricide, we studied killing of CARTS1 cells against T cells. A co-culture of CARTS1 cells with CD4+ or CD8+ T cells did not reveal any killing of T cells. Next, we tested CARTS1 cells in our JeKo-1 xenograft model. Treatment of tumor bearing mice with 5x106 CARTS1 cells resulted in complete durable remission (up to 60 days post treatment) of the tumor (p=0.0016), prolonged overall survival (Figure 1), and enhanced T cell proliferation at day 21 post treatment (p=0.0026), compared to control UTD mice.
Finally, we aimed to study whether TRAILshort targeting with CART cells impacts CART19 functions. Here, we generated CART cells which are dual targeting CD19 and TRAILshort. We tested the dual CART cells in our NSG mouse model. Mice were randomized to treat with CARTS1 cells, dual CD19/TRAILshort CART cells, CART19 cells or control UTD. Treatment with dual CART cells led to significantly less tumor burden as early as 15 days after CART cell injection (p <0.0001, p= 0.0044, p=0.0002 compared to UTD, CART19 and CARTRAILshort, respectively), and to prolonged overall survival (Figure 2).
Our findings demonstrate that targeting TRAILshort, a dominant negative TRAIL splice variant, either with monoclonal antibodies or CART cells enhances CART cell effector functions in preclinical B cell malignancies models.
Disclosures
Sakemura:Humanigen: Patents & Royalties. Kenderian:Tolero: Research Funding; Juno/BMS: Consultancy, Research Funding, Speakers Bureau; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Morphosys: Research Funding; Life Engine: Current holder of stock options in a privately-held company; Lentigen: Research Funding; Viracta/Sunesis: Research Funding; LEAH Labs: Current holder of stock options in a privately-held company, Research Funding; MustangBio: Patents & Royalties; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Mettaforge: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal