Abstract
Introduction: For patients with acute lymphoblastic leukemia (ALL) undergoing allogeneic hematopoietic stem cell transplantation (HCT) in first complete remission (CR1), measurable residual disease (MRD) prior to HCT is strongly associated with post-HCT relapse rates and overall survival (OS). However, for a growing number of patients with ALL, HCT is deferred until the second remission (CR2) or beyond. It is unclear whether pre-transplant MRD assessment is also prognostic in the setting of HCT beyond CR1.
Methods: We performed a single center retrospective study to test the hypothesis that pre-transplant MRD positivity is associated with outcomes in patients receiving HCT in CR2 or beyond for ALL. MRD was measured by 6-color flow cytometry with a sensitivity of 0.01%. Consecutive pediatric and adult patients who underwent first allogeneic HCT between the years 2004-2021 for ALL in CR2 or beyond were included.
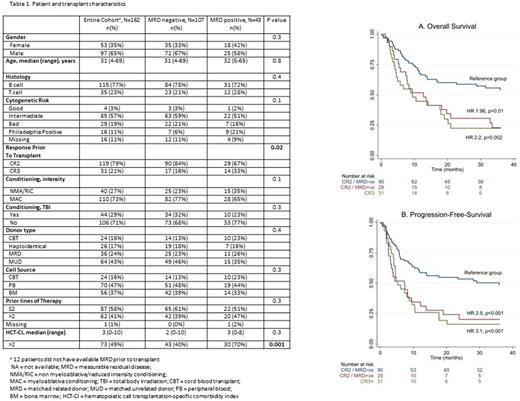
Results: We identified 162 patients (Table 1), 123 (76%) with B-ALL and the remainder with T-ALL. The majority (80%, n=129) of patients were in CR2 at the time of HCT, the remainder in third CR or beyond (CR3+). One hundred and five (65%) patients were male and median age at transplant was 31 (range 4-69) years. Graft source was either matched unrelated donor (MUD) (43%, n=69), matched sibling donor (MSD) (24%, n=39), haploidentical (18%, n=29) or cord blood transplantation (CBT) (15%, n=25). Pre-HCT MRD status was determined for 150 (93%) of patients and of those was positive in 43 (26%). Subsequent analyses were limited to the subset of patients with known MRD status. There was no statistical difference in characteristics according to MRD status except for higher proportion of CR3+ patients (33% vs. 16%, p=0.02) and comorbidity index >2 (70% vs. 40%, p=0.001) in MRD positive vs. negative patients. The median follow-up in surviving patients was 62 (range 20-104) and 47 (range: 2-171) months in MRD positive and negative patients, respectively. In multivariate analysis (MVA) evaluating transplantation outcomes at 3 years, pre-HCT MRD positivity was associated with a higher rate of disease relapse (HR 1.9, 95% CI 1.1-3.6; p=0.02); but it had no impact on non-relapse mortality (NRM) (HR 0.8, 95% CI 0.4-1.8; p=0.6). Subset analyses showed that these trends were consistent for patients receiving HCT in CR2 or CR3+; however the impact on disease relapse was more pronounced for patients in CR2 at HCT. MRD positivity was also associated with less favorable overall (HR 1.96, 95% CI 1.2-3.3; p=0.01) and progression-free survival (PFS) (HR 2.5, 95% CI 1.5-4.2; p<0.001) for patients receiving HCT in CR2. MRD was not associated with OS (HR 0.9, 95% CI 0.4-2, p=0.8) or PFS (HR 0.8, 95% CI 0.3-1.7, p=0.5) for patients receiving HCT in CR3+. As shown in Figure 1 A-B, OS and PFS were significantly superior for MRD negative patients receiving HCT in CR2. OS and PFS for MRD positive patients in CR2 were comparable to patients receiving HCT in CR3+.
Conclusions: Pre-transplant MRD positivity is predictive of disease relapse in patients undergoing HCT for ALL in CR2 or beyond; however, the survival advantage associated with MRD negativity maybe limited to patients undergoing HCT in CR2.
Disclosures
Popat:Bayer: Research Funding; Iovance: Consultancy; Incyte: Research Funding; Abbvie: Research Funding; Novartis: Research Funding. Alousi:Prolacta: Consultancy; Sanofi / Kadmon: Honoraria; Mallinkrodt: Honoraria; Incyte: Honoraria, Research Funding; Genetech: Consultancy. Oran:ASTEX: Research Funding; AROG: Research Funding. Short:Pfizer: Consultancy; Amgen: Consultancy, Honoraria; Novartis: Consultancy; Astellas: Research Funding; Stemline Therapeutics: Research Funding; AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Research Funding. Ravandi:AstraZeneca: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Xencor: Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Novartis: Consultancy. Champlin:Cell Source Inc.: Research Funding; Bluebird: Other: Data Safety Monitoring Board; Actinium: Consultancy; General Oncology: Other: Data Safety Monitoring Board; Kadmon: Consultancy; Johnson &Johnson: Consultancy; Omeros: Consultancy. Shpall:NY blood center: Consultancy; Bayer: Honoraria; Takeda: Patents & Royalties; Fibroblasts and FibroBiologics: Consultancy; Affimed: Other: License agreement; Navan: Consultancy; adaptimmune: Consultancy; axio: Consultancy. Kebriaei:Pfizer: Consultancy; Jazz: Consultancy; Amgen: Research Funding; Kite: Consultancy; Ziopharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal