Abstract
Background: Proteasome inhibition by bortezomib (BTZ) has previously been shown to be beneficial for a subset of patients with relapsed/refractory acute leukemia. Several (pre)clinical studies showed that relatively high immunoproteasome expression (β1i and β5i) may serve as a biomarker for BTZ-sensitivity and aid to select patients that may respond well to BTZ-containing therapy (Cloos J et al. Cancer Metastasis Rev. 2017). In this study we investigated whether we could confirm these findings in newly diagnosed T-cell acute lymphoblastic leukemia patients (T-ALL) enrolled in the COG-AALL1231 phase 3 clinical trial (Teachey DT et al. J Clin Oncol 2022). In that trial, patients in treatment arm A received augmented Berlin-Frankfurt-Münster (aBFM) therapy, while for those in treatment arm B, BTZ was added to the aBFM regimen.
Methods: Baseline immuno- and constitutive proteasome subunit expression was determined in bone marrow samples of 77 standard and intermediate risk T-ALL patients using Western blot. To correct for potential variation between blots, two cell lines were included as internal quantification controls, i.e. the human (BTZ-sensitive) T-ALL cell line CCRF-CEM (CEM-WT) and its subline (CEM-BTZ200) with acquired resistance to 200 nM BTZ. Protein bands were quantified and calculated relative to β-actin and the subunit expression of CEM-WT. Mann-Whitney U and log-rank tests were used for between group comparisons of proteasome subunit expressions and event free survival (EFS), respectively. Induction failure, relapse and death (induction and remission) were considered as EFS events.
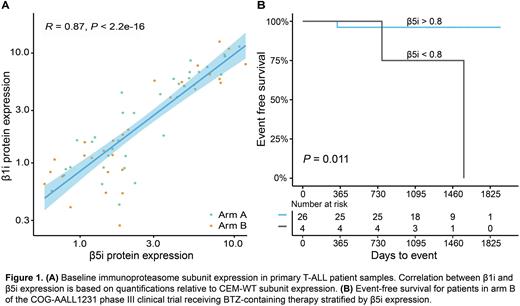
Results: In the bone marrow samples, a very strong correlation was observed between immunoproteasome β5i and β1i subunit expression (R=0.9, P<0.0001, Figure 1A). The correlation for constitutive subunits was R=0.7, P<0.0001. Interestingly, females had significantly higher β1i and β5i immunoproteasome levels compared to males with medians of 5.1 (range: 0.9-18.7) and 1.6 (0.3-17.9) respectively for the β1i subunit (P =0.05), and medians of 4.8 (1.9-16.1) and 1.8 (0.6-10.8) respectively for the β5i subunit (P =0.0084). Three year EFS was used as a measure for response to therapy. In patients treated with the BTZ-containing therapy (arm B), higher immunoproteasome expression levels (>0.8, based on Max-stat analysis) correlated with an improved EFS (P=0.034 for β1i and P=0.011 for β5i, Figure 1B). In contrast, in treatment arm A, higher immunoproteasome expression levels were associated with a relatively poor EFS, although this difference was not statistically significant likely due to low patient numbers in the low immunoproteasome subunit expression group in arm A (β1i N=5 and β5i N=1, for arm B β1i N=6 and β5i N=4). No other factors, including age, ethnicity, and gender, were found to be significantly associated with EFS.
Discussion: These results support our hypothesis that patients with higher immunoproteasome expression levels are responsive to proteasome inhibitors such as BTZ, with better EFS after BTZ-containing therapy. When comparing the patients with high immunoproteasome expression of arm A versus arm B, the latter displays a better EFS (3 years EFS 96% for arm B vs 80% for arm A, P=0.08). Although not statistically significant, this factor may be indicative of a beneficial effect of BTZ-treatment in this subgroup of patients. The limitation of this study is the relatively low number of samples in each subgroup, in particular the patients with low expression of immunoproteasome subunits. Since most patients show a high level of immunoproteasome, only the few with low expression might not benefit from BTZ treatment and may be allocated for alternative strategies. The relevance of the immunoproteasome for BTZ sensitivity in these samples will be further examined by proteasome subunit catalytic activity measurements with subunit-specific substrates and other known factors contribution to diminished BTZ sensitivity (Niewerth et al. Drug Resist Updates 2015).
Disclosures
Teachey:BEAM Therapeutics: Consultancy; Sobi: Consultancy. Zweegman:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cloos:Takeda: Research Funding; Helsinn: Other: MRD assessments; Navigate: Patents & Royalties: Royalties for MRD analyses; Merus: Other: MRD assessments, Research Funding; Janssen: Research Funding; Novartis: Consultancy, Other: MRD assessments, Research Funding; DC-One: Other: MRD assessments, Research Funding; Genentech: Research Funding; Astellas: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal