Abstract
IntroductionIKZF1 codes Ikaros, which is a member of a family of zinc finger proteins that is required for normal lymphopoiesis. IKZF1 alterations in cases of B-cell acute lymphoblastic leukemia (B-ALL) were mainly analyzed in a pediatric cohort and showed an association with poor prognosis. In adults IKZF1 alterations are found in approximately 50% of B-ALL cases, and 65% of cases with BCR-ABL-positive B-ALL. IKZF1 deletion have several different types of deletions. Deletion of exons 4 to 7 (Δ4-7) leads to nonfunctional isoforms that do not bind DNA. However, they can still dimerize with residual normal isoforms, suppressing the function of the normal Ikaros protein expressed from the nondeleted allele, a so-called "dominant-negative” (DN) effect. Other deletions lead to loss of function (LOF) of Ikaros. The impact of DN and LOF type of IKZF1 deletion on prognosis in adult B-ALL patients has not been elucidated. We evaluated the impacts of IKZF1 deletion types on overall survival (OS) in our cohort.
Patients and Methods
Hokkaido Leukemia Net (HLN) is prospective cohort study collecting acute leukemia samples from hospitals of North Japan Hematology Study Group (NJHSG) covering Hokkaido, the northernmost island of Japan. In this study, we focused on newly diagnosed adult B-ALL patients. Bone marrow or peripheral blood samples from newly diagnosed B-ALL patients were screened by a fluorescence in situ hybridization (FISH) probe for detecting IKZF1 deletion (Hashiguchi J. J Mol Diagn. 2018). Then DNA specimens from IKZF1 deletion-positive patients were screened for the 4 most common intragenic IKZF1 deletions, Δ4-7, Δ2-7, Δ2-8 and Δ4-8, by polymerase chain reaction (PCR). Samples in which IKZF1 deletion was detected by FISH but in which the types of deletions were not identified were defined as "other". We categorized the patients into a DN group that had Δ4-7, LOF and other group, and wildtype (WT) group based on the types of IKZF1 deletion. This study was conducted in accordance with the Helsinki Declaration and was approved by the institutional review boards.
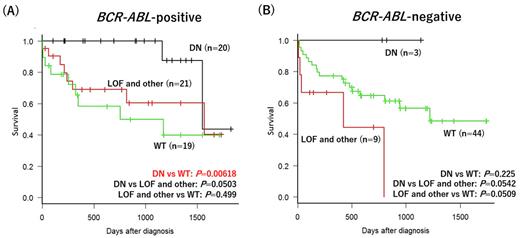
Results Overall, 116 patients with newly diagnosed B-ALL were enrolled in HLN between August 2014 and December 2021 (age range, 17-90 years; median age ,59 years; 55 males and 61 females; 60 BCR-ABL-positive patients and 56 BCR-ABL-negative patients). IKZF1 deletion was detected in 53 cases including 6 cases with bi-allelic deletion. IKZF1 deletions were detected in 68.3% of BCR-ABL-positive B-ALL cases and 21.4% of BCR-ABL-negative B-ALL cases. DN/LOF and other ratio was larger in BCR-ABL-positive B-ALL cases than in BCR-ABL-negative B-ALL cases (20/21 vs 3/9). In patients with BCR-ABL-positive B-ALL, patients with the DN type of IKZF1 deletion had a significantly longer OS than that in the WT group. The 3-year OS rates in the DN, LOF and other, and WT groups were 100%, 60.4%, and 49.9%, respectively (Figure A). Distribution of age, sex, percentage of allogenic hematopoietic stem cell transplantation (allo-HSCT) were not significantly different between the three groups. Survival was analysed by univariate analysis including age, sex, WBC counts, complex karyotype, IKZF1 deletion type and allo-HSCT. Age≥65 was associated with poor OS (P=0.0078) and DN type of IKZF1 deletion was associated with favorable OS (P=0.026). Multivariate analysis showed that older age and DN type of IKZF1 deletion were independently associated with OS (age≥65: HR, 3.510; 95% CI, 1.327 to 9.289, P=0.011; DN type of IKZF1 deletion: HR, 0.200; 95% CI, 0.046 to 0.884, P=0.034; Cox regression). In patients with BCR-ABL-negative B-ALL, there was no statistically significant difference in OS between the three groups, whereas the LOF and other group showed a dismal prognosis. The 3-year OS rates in the DN, LOF and other, and WT groups were 100%, 0%, and 56.5%, respectively (Figure B).
Conclusion The prognostic impact of IKZF1 deletion might differ in BCR-ABL-positive and BCR-ABL-negative B-ALL cases and might also depend on the type of deletion. DN type of IKZF1 deletion is independently associated with favorable prognosis in adult BCR-ABL-positive B-ALL patients.
Disclosures
Kondo:Alexion Pharma: Honoraria; PharmaEssentia Japan: Honoraria; Chugai Pharmaceutical: Honoraria. Teshima:NIPPON SHINYAKU: Research Funding; Janssen: Other: Manuscript preparation; Pfizer: Honoraria; TEIJIN PHARMA: Research Funding; Fuji Pharma: Research Funding; Bristol-Myers Squibb: Honoraria; Astellas: Research Funding; Chugai: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Manuscript preparation, Research Funding; Merck Sharp & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Luca Science Inc.: Research Funding; Sanofi: Research Funding; Kyowa Kirin: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal