Key Points
Use of the rhIL-22 dimer F-652 with systemic steroids appeared safe and was associated with a high response rate in newly diagnosed GI GVHD.
Patients responding after tissue-targeted therapy with F-652 and corticosteroids demonstrated an expansion of healthy commensal GI flora.
Abstract
Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality following allogeneic hematopoietic transplantation. In experimental models, interleukin-22 promotes epithelial regeneration and induces innate antimicrobial molecules. We conducted a multicenter single-arm phase 2 study evaluating the safety and efficacy of a novel recombinant human interleukin-22 dimer, F-652, used in combination with systemic corticosteroids for treatment of newly diagnosed lower gastrointestinal acute GVHD. The most common adverse events were cytopenias and electrolyte abnormalities, and there were no dose-limiting toxicities. Out of 27 patients, 19 (70%; 80% confidence interval, 56%-79%) achieved a day-28 treatment response, meeting the prespecified primary endpoint. Responders exhibited a distinct fecal microbiota composition characterized by expansion of commensal anaerobes, which correlated with increased overall microbial α-diversity, suggesting improvement of GVHD-associated dysbiosis. This work demonstrates a potential approach for combining immunosuppression with tissue-supportive strategies to enhance recovery of damaged mucosa and promote microbial health in patients with gastrointestinal GVHD. This trial was registered at www.clinicaltrials.gov as NCT02406651.
Introduction
Acute graft-versus-host disease (aGVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic/stem cell transplantation (allo-HCT),1-4 and aGVHD affecting the lower gastrointestinal (GI) tract is particularly associated with an increased risk of transplant-related mortality (TRM).3-6 Current approaches for prophylaxis and treatment of GVHD are largely immunosuppressive in nature, impairing immune reconstitution and increasing the risks of infection and malignant relapse.7-9 Corticosteroids are the standard up-front treatment for grades 2 to 4 aGVHD, with published response rates ranging from 40% to 70%.3,4 There are currently no approved drugs for improving standard corticosteroid responses in the up-front treatment setting.
Interleukin-22 (IL-22) is a tissue-protective IL-10–family cytokine produced by several populations of innate and adaptive immune cells.10 It has been shown to promote mucosal healing and improve intestinal barrier function through nonimmunosuppressive mechanisms. IL-22 signals directly to the intestinal epithelium, supporting enterocyte survival and epithelial regeneration.11,12 In experimental models, aGVHD led to reduced intestinal stem cell (ISC) frequency and loss of host-derived IL-22–producing cells.11,13-15 Furthermore, IL-22 deficiency resulted in increased GI pathology, loss of epithelial integrity, and substantial GVHD-associated mortality after experimental bone marrow transplantation (BMT).11 ISCs express the IL-22 receptor, and murine allogeneic BMT recipients treated in vivo with exogenous IL-22 demonstrated greater numbers of ISCs, reduced GI aGVHD pathology, and improved survival.12 In addition, IL-22 is a potent inducer of antimicrobial molecules, including β-defensins, regenerating islet-derived protein 3α (REG3α), and mucins.16-19 IL-22 deficiency can result in alterations of the gut microbiota,20 and the intestinal microbiome itself can be a modulator of alloreactivity and GVHD.21-23
Expression of the IL-22 receptor is largely restricted to nonhematopoietic cells.10 Given this and its mechanism of action, IL-22 administration has the potential to improve clinical GVHD treatment responses without exacerbating posttransplant immunodeficiency. However, native recombinant cytokines such as IL-22 have short half-lives of less than 2 hours in vivo, which impedes clinical applications.24 F-652 is a novel recombinant human (rh) IL-22 molecule consisting of a human IL-22 dimer at the N-terminus and human immunoglobulin G2 Fc at the C-terminus, providing improved pharmacologic stability. It has an extended half-life ranging from 39.4 to 206 hours in healthy people treated with escalating doses of the drug. F-652 was well tolerated in these patients, with mild to moderate xerosis and xerophthalmia being the most common adverse events (AEs).24 Here, we report the results of a multicenter phase 2 study of F-652 administration along with systemic corticosteroids for initial treatment of lower GI aGVHD.
Methods
Full methodology
Full details of the methods, including study design, correlative analyses, and statistics, are included in the supplemental Full Methods, available on the Blood website.
Study design
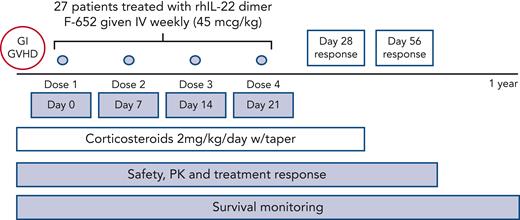
This was an open-label, single-cohort, multicenter phase 2 study sponsored by Evive Biotechnology (Shanghai) Ltd (formerly Generon [Shanghai] Corporation Ltd) and conducted at Memorial Sloan Kettering Cancer Center, University of Texas MD Anderson Cancer Center, and City of Hope National Medical Center. Enrollment commenced in May 2016 and was completed in March 2019. The protocol and informed consents were approved by the institutional review boards of all participating institutions. All patients signed informed consents in accordance with the Declaration of Helsinki. The primary objectives of the study were to evaluate the safety, pharmacokinetics (PK), and GVHD treatment response following administration of F-652 and corticosteroids. The primary efficacy endpoint was day-28 treatment response. Secondary objectives included assessment of day-56 treatment response and 1-year survival. Treatment responses were categorized as complete response (CR), very good partial response (VGPR), partial response (PR), and treatment failure. Correlative studies included peripheral blood cytokine and biomarker analyses as well as stool microbiota assessment. Treatment-emergent AEs were defined as AEs for which the start date occurred on or after the date that F-652 treatment began.
Patients
Full inclusion and exclusion criteria are listed in supplemental Table 1. Eligible patients were required to be aged between 18 to 80 years and have newly diagnosed stage 1 to stage 4 aGVHD of the lower GI tract. Endoscopic biopsy was required for enrollment, although F-652 treatment could be initiated empirically prior to determination of the biopsy results. Patients whose biopsy results returned with evidence of active infection or otherwise did not support a diagnosis of GI GVHD were removed from the trial and replaced, although they continued to be evaluable for toxicity. Patients received antibiotic prophylaxis according to their institutional guidelines.
Treatment
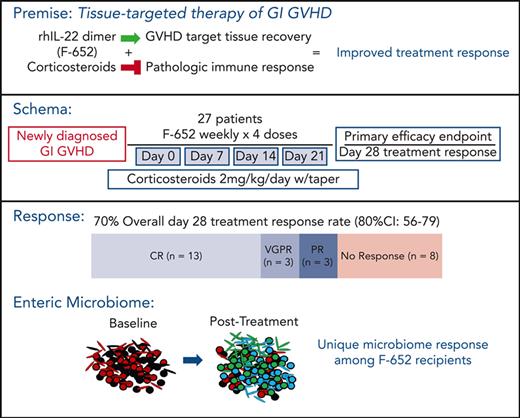
Participants were administered F-652 intravenously at a dose of 45 μg/kg weekly, for up to 4 doses, along with concurrent systemic corticosteroids (Figure 1). Patients who had progression of aGVHD after 7 days of therapy (prior to dose #2) or no response/stable symptoms after 14 days of therapy (prior to dose #3) were considered to be treatment failures and discontinued from further treatment. The dose and schedule of F-652 administration were based on results from a phase 1 study performed in healthy volunteers.24 Here, F-652 treatment was initiated within 5 days of starting systemic corticosteroids. At the time of enrollment, all patients received prednisone (or IV equivalent) at a dose of 2 mg/kg per day, which was mandated to be continued for a minimum of 3 days following the first dose of F-652. Corticosteroids could then be tapered per institutional practice, although a minimum dose of 0.25 mg/kg per day of prednisone (or IV equivalent) was still required through day 28 of therapy.
Protocol schema. The rhIL-22 dimer F-652 was administered weekly for a total of 4 doses in combination with systemic corticosteroids to patients with newly diagnosed GI aGVHD. Patients were monitored for safety, drug PK, clinical endpoints including day-28 aGVHD response rates, and correlative analyses including fecal microbiota composition.
Protocol schema. The rhIL-22 dimer F-652 was administered weekly for a total of 4 doses in combination with systemic corticosteroids to patients with newly diagnosed GI aGVHD. Patients were monitored for safety, drug PK, clinical endpoints including day-28 aGVHD response rates, and correlative analyses including fecal microbiota composition.
Statistics
Differences in categorical variables were evaluated using χ2 or Fisher exact test as appropriate, whereas differences in categorical and continuous variables were evaluated using Wilcoxon rank sum test. Cumulative incidence functions were used to estimate the incidence of GVHD and TRM. The competing risks for each outcome were death or relapse for GVHD and relapse for TRM. Progression-free survival and overall survival were estimated using the Kaplan-Meier method. A Simon’s 2-stage optimal design was implemented to distinguish between an unpromising response rate of 35% and a promising response rate of 60% for day-28 treatment response, which was used for determination of the sample size. The type I and type II errors were both set at 0.10. PK comparisons based on response status were estimated by a Wilcoxon rank sum test.
Results
Patient demographics
Twenty-seven patients with newly diagnosed, biopsy-proven lower GI aGVHD were enrolled and monitored for treatment response (Table 1). Three additional patients who received 1 dose of F-652 were excluded from further treatment owing to incompatible findings on biopsy (1 negative for GVHD and 2 positive for infection) but placed under continued toxicity monitoring (supplemental Figure 1). AEs were monitored for up to 56 days following the first dose of F-652, and all surviving patients were followed for at least 1 year after study enrollment. Assessment of antibiotic exposure indicated that patients in this study were treated with a wide range of broad-spectrum antibiotics prior to, during, and after F-652 administration (supplemental Table 2).
Patient demographics (n = 27)
| Characteristic . | Patients . |
|---|---|
| Median age, y (range) | 55 (22-72) |
| Male sex, n (%) | 14 (52) |
| Diagnosis, n (%) | |
| Acute leukemia/MDS | 20 (74) |
| Lymphoma | 5 (18) |
| CML/MM | 2 (3) |
| MA conditioning, n (%) | |
| TBI-based | 4 (15) |
| Chemotherapy-based | 7 (26) |
| RI conditioning, n (%) | |
| TBI-based | 4 (15) |
| Chemotherapy-based | 12 (44) |
| Donor, n (%) | |
| MRD | 4 (15) |
| MUD/MMUD∗ | 13/6 (70) |
| Haploidentical | 4 (15) |
| HLA match, n (%) | |
| 8/8 | 17 (63) |
| 7/8 | 2 (7) |
| <7/8 | 8 (30) |
| Stem cell source, n (%) | |
| BM | 4 (15) |
| PBSC | 19 (70) |
| Cord blood | 4 (15) |
| GVHD prophylaxis, n (%) | |
| CNI ± MTX ± sirolimus | 14 (52) |
| CNI/MMF | 5 (18.5) |
| PTCy/Tacrolimus ± MMF | 8 (29.5) |
| LGI GVHD stage, n (%) | |
| 1 | 9 (33) |
| 2 | 4 (15) |
| 3-4 | 14 (52) |
| Characteristic . | Patients . |
|---|---|
| Median age, y (range) | 55 (22-72) |
| Male sex, n (%) | 14 (52) |
| Diagnosis, n (%) | |
| Acute leukemia/MDS | 20 (74) |
| Lymphoma | 5 (18) |
| CML/MM | 2 (3) |
| MA conditioning, n (%) | |
| TBI-based | 4 (15) |
| Chemotherapy-based | 7 (26) |
| RI conditioning, n (%) | |
| TBI-based | 4 (15) |
| Chemotherapy-based | 12 (44) |
| Donor, n (%) | |
| MRD | 4 (15) |
| MUD/MMUD∗ | 13/6 (70) |
| Haploidentical | 4 (15) |
| HLA match, n (%) | |
| 8/8 | 17 (63) |
| 7/8 | 2 (7) |
| <7/8 | 8 (30) |
| Stem cell source, n (%) | |
| BM | 4 (15) |
| PBSC | 19 (70) |
| Cord blood | 4 (15) |
| GVHD prophylaxis, n (%) | |
| CNI ± MTX ± sirolimus | 14 (52) |
| CNI/MMF | 5 (18.5) |
| PTCy/Tacrolimus ± MMF | 8 (29.5) |
| LGI GVHD stage, n (%) | |
| 1 | 9 (33) |
| 2 | 4 (15) |
| 3-4 | 14 (52) |
BM, bone marrow; CML, chronic myeloid leukemia; CNI, calcineurin inhibitor; LGI, lower gastrointestinal; MA, myeloablative; MDS, myelodysplastic syndrome; MM, multiple myeloma; MMF, mycophenolate mofetil; MMUD, mismatched-unrelated donor; MRD, matched-related donor; MTX, methotrexate; MUD, matched-unrelated donor; PBSC, peripheral blood stem cell; PTCy, post-transplant cyclophosphamide; RI, reduced intensity; TBI, total body irradiation.
Includes 4 patients who received cord blood grafts.
Median age at transplant was 55 (range, 22-72) years, and the most common malignant diagnoses were acute leukemia and myelodysplastic syndrome (Table 1). Most patients received a peripheral blood graft from an 8/8 HLA-matched donor, and 30% percent received an alternative donor graft (haploidentical or umbilical cord). Prior to enrollment, a calcineurin inhibitor plus methotrexate or mycophenolate mofetil was used as GVHD prophylaxis in most patients; 8 of 27 patients received posttransplant cyclophosphamide-based GVHD prophylaxis. Lower GI aGVHD occurred at a median onset of 27 (range, 16-126) days following allo-HCT. Stage 1, 2, 3, or 4 lower GI aGVHD was present in 9 (33%), 4 (15%), 7 (26%), and 7 (26%) patients, respectively. Most patients had isolated GI GVHD (all with lower gut involvement ± upper gut involvement), with 3 patients having concurrent skin involvement. No patients had concurrent liver involvement at enrollment. According to Minnesota scoring,4 12 patients had standard-risk aGVHD and 15 had high-risk aGVHD.
Pharmacokinetics and safety of rhIL-22 administration in patients with acute GVHD
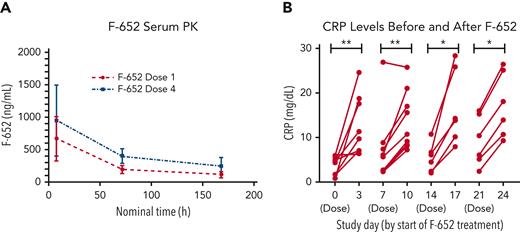
Twenty-eight patients underwent PK evaluation following a single IV infusion of F-652. Patients who had progression of aGVHD after 7 days of therapy or no response/stable symptoms after 14 days of therapy did not receive all planned F-652 doses (n = 7). PK profiling included measurements at 8 hours, 72 hours, and 7 days following the first and fourth F-652 infusions (administered on day 0 and 21, respectively). F-652 PK levels indicated rapid serum detection and distribution following both the first and fourth doses (Figure 2A).
PK and pharmacodynamics monitoring. (A) PK analyses showed detectable F-652 levels at 8 hours, 72 hours, and 7 days following the first and last doses of the rhIL-22 dimer. (B) Measurement of C-reactive protein (CRP) levels prior to administering F-652 and 3 days after administration. F-652 was administered on stay days 0 (dose 1), 7 (dose 2), 14 (dose 3), and 21 (dose 4). ∗P < .05, ∗∗P < .01.
PK and pharmacodynamics monitoring. (A) PK analyses showed detectable F-652 levels at 8 hours, 72 hours, and 7 days following the first and last doses of the rhIL-22 dimer. (B) Measurement of C-reactive protein (CRP) levels prior to administering F-652 and 3 days after administration. F-652 was administered on stay days 0 (dose 1), 7 (dose 2), 14 (dose 3), and 21 (dose 4). ∗P < .05, ∗∗P < .01.
IL-22 induces an acute phase response with up-regulation of acute phase proteins including CRP.25,26 Accordingly, a subset of patients had pre- and postdose CRP levels measured as a pharmacodynamic assessment. CRP levels were mildly elevated prior to F-652 infusion in this aGVHD patient cohort. Nonetheless, the median CRP level increased following each infusion of F-652 (Figure 2B). Recombinant human (rh) IL-22 was thus administered with a measurable in vivo response in patients with aGVHD.
Thirty patients were evaluable for drug toxicity, including the 3 patients who received a single dose of F-652 prior to identification of incompatible histopathology. Twenty-seven patients (87%) experienced at least 1 AE regardless of attribution, and 17 patients (57%) had an AE potentially attributable to the study drug (Table 2). Most AEs were grade 1 or 2, including anemia (40%), thrombocytopenia (43%), lymphopenia (37%), hypokalemia (50%), hypomagnesemia (44%), xerostomia (20%), xeropthalmia (13%), and skin xerosis (23%), and 70% of these events had resolved by study day 56. Twenty-two possibly, probably, or definitely drug-related treatment-emergent AEs grade 3 or above occurred in 9 participants during the study, the most common being cytopenias and liver function test abnormalities. Of those, 45% resolved or were recovering by study day 56. The most common unresolved and potentially drug-related serious AEs included leukopenia and thrombocytopenia (Table 3). Five patients experienced relapse or progression of their malignant disease between 94 to 343 days following enrollment. The cumulative incidence of disease relapse at 1 year was 18.5% (95% CI, 4%-33%), with 3 deaths attributable to relapse, which is in line with historical data for this patient population.27 Out of the 5 patients who relapsed, 4 had a day-28 GI aGVHD treatment response, and 1 had treatment failure. One patient had stage 2 skin squamous cell carcinoma (pT2N0M0) diagnosed approximately 9 months after enrollment and was treated with Mohs surgery and adjuvant radiation. No patients had their treatment dose reduced.
Treatment-emergent adverse events reported in ≥10% of patients
| . | Any grade, n (%) . | Drug-related grade ≥3, n (%) . |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Anemia | 12 (40) | |
| INR increased | 5 (17) | |
| Lymphopenia | 11 (37) | |
| Neutropenia | 6 (20) | |
| Thrombocytopenia | 13 (43) | 7 (23) |
| Prolonged aPTT | 3 (10) | |
| Leukopenia | 9 (30) | 3 (10) |
| Cardiovascular | ||
| Hypertension | 6 (20) | |
| ENMT | ||
| Xerostomia | 6 (20) | |
| Gastrointestinal | ||
| Abdominal pain | 5 (17) | |
| Anorexia | 3 (10) | |
| Elevated alkaline phosphatase | 10 (33) | |
| Elevated alanine aminotransferase | 8 (27) | |
| Elevated aspartate aminotransferase | 6 (20) | |
| Hyperbilirubinemia | 8 (27) | |
| Nausea | 3 (10) | |
| Vomiting | 6 (20) | |
| Weight gain | 3 (10) | |
| Weight loss | 3 (10) | |
| General | ||
| Chills | 3 (10) | |
| Edema limbs | 4 (13) | |
| Xeropthalmia | 4 (13) | |
| Fatigue | 5 (17) | |
| Fever | 4 (13) | |
| Noncardiac chest pain | 3 (10) | |
| Infection | ||
| Catheter-related infection | 3 (10) | |
| Sepsis | 3 (10) | |
| Metabolic | ||
| Hyperkalemia | 6 (20) | |
| Hyperglycemia | 11 (37) | |
| Hypermagnesemia | 5 (17) | |
| Hypernatremia | 4 (13) | |
| Hypertriglyceridemia | 5 (17) | |
| Hypoalbuminemia | 10 (33) | |
| Hypocalcemia | 8 (27) | |
| Hypoglycemia | 6 (20) | |
| Hypokalemia | 15 (50) | |
| Hypomagnesemia | 10 (33) | |
| Hyponatremia | 9 (30) | |
| Hypophosphatemia | 10 (33) | |
| Musculoskeletal | ||
| Generalized muscle weakness | 7 (23) | |
| Nervous system disorder | ||
| Dizziness | 5 (17) | |
| Dysgeusia | 3 (10) | |
| Tremor | 3 (10) | |
| Psychiatric disorders | ||
| Anxiety | 4 (13) | |
| Insomnia | 7 (23) | |
| Respiratory | ||
| Cough | 3 (10) | |
| Dyspnea | 5 (17) | |
| Epistaxis | 3 (10) | |
| Other | ||
| Pruritus | 5 (17) | |
| Xerosis | 7 (23) |
| . | Any grade, n (%) . | Drug-related grade ≥3, n (%) . |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Anemia | 12 (40) | |
| INR increased | 5 (17) | |
| Lymphopenia | 11 (37) | |
| Neutropenia | 6 (20) | |
| Thrombocytopenia | 13 (43) | 7 (23) |
| Prolonged aPTT | 3 (10) | |
| Leukopenia | 9 (30) | 3 (10) |
| Cardiovascular | ||
| Hypertension | 6 (20) | |
| ENMT | ||
| Xerostomia | 6 (20) | |
| Gastrointestinal | ||
| Abdominal pain | 5 (17) | |
| Anorexia | 3 (10) | |
| Elevated alkaline phosphatase | 10 (33) | |
| Elevated alanine aminotransferase | 8 (27) | |
| Elevated aspartate aminotransferase | 6 (20) | |
| Hyperbilirubinemia | 8 (27) | |
| Nausea | 3 (10) | |
| Vomiting | 6 (20) | |
| Weight gain | 3 (10) | |
| Weight loss | 3 (10) | |
| General | ||
| Chills | 3 (10) | |
| Edema limbs | 4 (13) | |
| Xeropthalmia | 4 (13) | |
| Fatigue | 5 (17) | |
| Fever | 4 (13) | |
| Noncardiac chest pain | 3 (10) | |
| Infection | ||
| Catheter-related infection | 3 (10) | |
| Sepsis | 3 (10) | |
| Metabolic | ||
| Hyperkalemia | 6 (20) | |
| Hyperglycemia | 11 (37) | |
| Hypermagnesemia | 5 (17) | |
| Hypernatremia | 4 (13) | |
| Hypertriglyceridemia | 5 (17) | |
| Hypoalbuminemia | 10 (33) | |
| Hypocalcemia | 8 (27) | |
| Hypoglycemia | 6 (20) | |
| Hypokalemia | 15 (50) | |
| Hypomagnesemia | 10 (33) | |
| Hyponatremia | 9 (30) | |
| Hypophosphatemia | 10 (33) | |
| Musculoskeletal | ||
| Generalized muscle weakness | 7 (23) | |
| Nervous system disorder | ||
| Dizziness | 5 (17) | |
| Dysgeusia | 3 (10) | |
| Tremor | 3 (10) | |
| Psychiatric disorders | ||
| Anxiety | 4 (13) | |
| Insomnia | 7 (23) | |
| Respiratory | ||
| Cough | 3 (10) | |
| Dyspnea | 5 (17) | |
| Epistaxis | 3 (10) | |
| Other | ||
| Pruritus | 5 (17) | |
| Xerosis | 7 (23) |
aPTT, activated partial thromboplastin time; ENMT, ear, nose, mouth, and throat; INR, international normalized ratio.
Outcome status of possibly, probably, or definitely F-652–related treatment emergent adverse events
| Grade 1-2 (46 events, 16 participants) . | Resolved/recovering, n . | Not resolved, n . |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Neutropenia (n = 1) | 1 | 0 |
| ENMT | ||
| Xerostomia (n = 4) | 3 | 1 |
| Dysgeusia (n = 2) | 1 | 1 |
| Gastrointestinal | ||
| LFT abnormalities (n = 13) | 11 | 2 |
| General | ||
| Chills (n = 4) | 4 | 0 |
| Malaise (n = 1) | 1 | 0 |
| Xeropthalmia (n = 1) | 1 | 0 |
| Cardiac | ||
| First-degree atrioventricular block (n = 1) | 0 | 1 |
| Metabolic | ||
| Hypercholesterolemia (n = 2) | 0 | 2 |
| Hypertriglyceridemia (n = 2) | 1 | 1 |
| Hyponatremia (n = 1) | 1 | 0 |
| Hypophosphatemia (n = 1) | 0 | 1 |
| Nervous system disorder | ||
| Paresthesia (n = 1) | 1 | 0 |
| Other | ||
| Pruritus (n = 3) | 3 | 0 |
| Skin condition (n = 3)∗ | 3 | 0 |
| Xerosis (n = 5) | 0 | 5 |
| Urinary tract infection (n = 1) | 1 | 0 |
| Grade ≥3 (22 events, 9 participants) | ||
| Blood and lymphatic system disorders | ||
| Anemia (n = 2) | 1 | 1 |
| Leukopenia (n = 3) | 1 | 2 |
| Neutropenia (n = 1) | 1 | 0 |
| Lymphopenia (n = 2) | 1 | 1 |
| Thrombocytopenia (n = 7) | 2 | 5 |
| Gastrointestinal | ||
| LFT abnormalities (n = 4) | 2 | 2 |
| General | ||
| Chills (n = 1) | 1 | 0 |
| Metabolic | ||
| Hyponatremia (n = 2) | 1 | 1 |
| Grade 1-2 (46 events, 16 participants) . | Resolved/recovering, n . | Not resolved, n . |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Neutropenia (n = 1) | 1 | 0 |
| ENMT | ||
| Xerostomia (n = 4) | 3 | 1 |
| Dysgeusia (n = 2) | 1 | 1 |
| Gastrointestinal | ||
| LFT abnormalities (n = 13) | 11 | 2 |
| General | ||
| Chills (n = 4) | 4 | 0 |
| Malaise (n = 1) | 1 | 0 |
| Xeropthalmia (n = 1) | 1 | 0 |
| Cardiac | ||
| First-degree atrioventricular block (n = 1) | 0 | 1 |
| Metabolic | ||
| Hypercholesterolemia (n = 2) | 0 | 2 |
| Hypertriglyceridemia (n = 2) | 1 | 1 |
| Hyponatremia (n = 1) | 1 | 0 |
| Hypophosphatemia (n = 1) | 0 | 1 |
| Nervous system disorder | ||
| Paresthesia (n = 1) | 1 | 0 |
| Other | ||
| Pruritus (n = 3) | 3 | 0 |
| Skin condition (n = 3)∗ | 3 | 0 |
| Xerosis (n = 5) | 0 | 5 |
| Urinary tract infection (n = 1) | 1 | 0 |
| Grade ≥3 (22 events, 9 participants) | ||
| Blood and lymphatic system disorders | ||
| Anemia (n = 2) | 1 | 1 |
| Leukopenia (n = 3) | 1 | 2 |
| Neutropenia (n = 1) | 1 | 0 |
| Lymphopenia (n = 2) | 1 | 1 |
| Thrombocytopenia (n = 7) | 2 | 5 |
| Gastrointestinal | ||
| LFT abnormalities (n = 4) | 2 | 2 |
| General | ||
| Chills (n = 1) | 1 | 0 |
| Metabolic | ||
| Hyponatremia (n = 2) | 1 | 1 |
LFT, liver function test.
Skin condition includes erythema, peeling, and stage 2 skin squamous cell carcinoma.
Response rates following rhIL-22 therapy and systemic corticosteroids
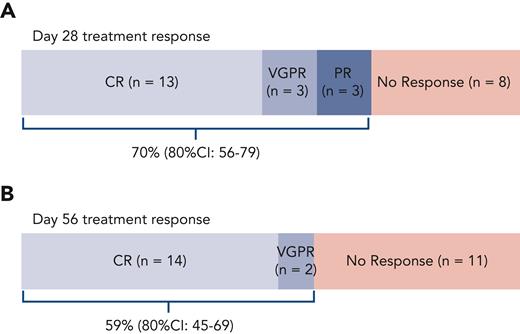
Out of 27 patients with evaluable aGVHD treated with F-652 and systemic corticosteroids, 19 (70%; 80% CI, 56%-79%) achieved a day-28 response in the lower GI tract (Figure 3A), including CRs (n = 13), VGPRs (n = 3), and PRs (n = 3). In these patients, stage 1, 2, 3, or 4 lower GI aGVHD were present in 9, 3, 4, and 3 patients, respectively, at enrollment. Out of 13 patients with lower GI stage 1 to 2 (grade 2) aGVHD, 12 patients achieved day-28 treatment responses; of the 14 patients with lower GI stage 3 to 4 (grade 3-4) aGVHD, 7 demonstrated day-28 treatment responses (supplemental Figure 2). Day-28 treatment responders thus included patients with a wide range of lower GI aGVHD severity at presentation, including 4 patients with stage 3 and 3 patients with stage 4 disease. Sixteen patients remained responders at day 56 (59%; 80% CI, 45%-69%), including 14 CRs and 2 VGPRs of lower GI aGVHD (Figure 3B).
Treatment response. Per study design, 27 patients were enrolled with biopsy-proven lower GI aGVHD and monitored for their response to treatment with F-652 (rhIL-22) and systemic corticosteroids. The study was powered to indicate a promising treatment response if at least 60% of patients responded with an improvement in GVHD staging at day 28 after F-652 initiation. (A) The primary efficacy endpoint was achieved with an overall day-28 treatment response of 70%. (B) The majority of patients remained responders by day 56.
Treatment response. Per study design, 27 patients were enrolled with biopsy-proven lower GI aGVHD and monitored for their response to treatment with F-652 (rhIL-22) and systemic corticosteroids. The study was powered to indicate a promising treatment response if at least 60% of patients responded with an improvement in GVHD staging at day 28 after F-652 initiation. (A) The primary efficacy endpoint was achieved with an overall day-28 treatment response of 70%. (B) The majority of patients remained responders by day 56.
Patients were followed for 1 year after the first dose of F-652. During the assessment period, 8 patients died of TRM for a cumulative 1-year TRM incidence of 30% (95% CI, 12%-47%). The primary cause of death for patients with TRM was GVHD for all but 1 patient who died of brain toxoplasmosis. Five patients who died of GVHD had either no response or had GVHD progression at study day 28, 1 had a day-28 PR, and 1 had a day-28 CR but subsequently progressed after day 56. The median time from study enrollment to death was 124.5 (range, 23-303) days. The 1-year overall survival and progression-free survival were 63% (95% CI, 45%-81%) and 52% (95% CI, 33%-71%), respectively.
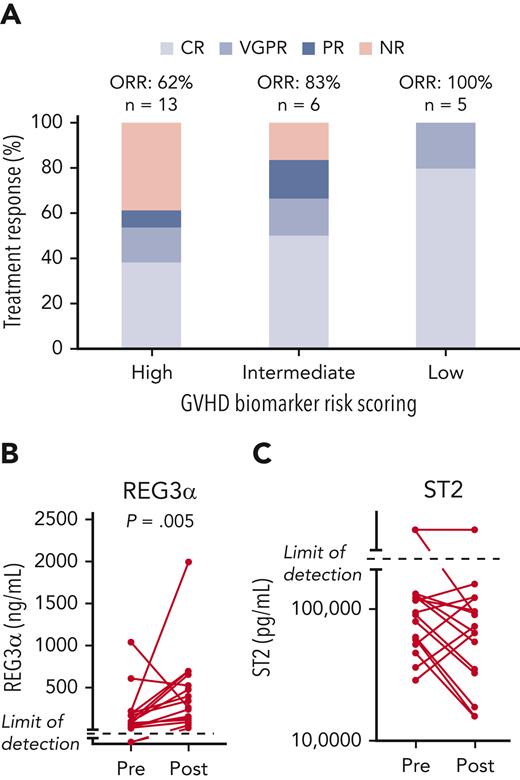
To better understand the differences between responders and nonresponders, we analyzed GVHD biomarker risk scores from study participants. The Mount Sinai Acute GVHD International Consortium GVHD risk scoring algorithm was performed based on the concentrations of the biomarkers REG3α and ST228 obtained from 24 study patients at the time of enrollment. Most patients had either high (n = 13) or intermediate (n = 6) risk scores, whereas 5 patients had low risk scores. Treatment responses were observed in 8 of 13 patients (62%) with high-risk, 5 of 6 patients (83%) with intermediate-risk, and 5 of 5 patients (100%) with low-risk biomarkers (Figure 4A). Of the 8 patients who died of TRM, 6 had high-risk GVHD biomarker scoring at aGVHD onset, and 1 had intermediate-risk aGVHD, with 1 not having samples available for biomarker analysis.
GVHD biomarker–based stratification of study participants. Twenty-four patients in the study underwent GVHD risk assessment per the Mount Sinai Acute GVHD International Consortium scoring algorithm of plasma GVHD biomarkers REG3α and ST2. (A) Treatment response according to GVHD biomarker risk score at enrollment (n = 24). The majority of study participants had high or intermediate risk scores (n = 19); low-risk disease represented the minority of patients under study (n = 5.) Day-28 responses were observed in all risk groups. (B) Assessment of REG3α levels before and after F-652 treatment in 18 patients with paired plasma samples taken at study enrollment and again after completion of F-652 treatment. REG3α levels were significantly greater posttreatment when compared with baseline measurements. (C) Assessment of ST2 levels before and after F-652 treatment in 19 patients with paired plasma samples taken at study enrollment and again after completion of F-652 treatment. ST2 levels showed a mixed response posttreatment when compared with baseline measurements.
GVHD biomarker–based stratification of study participants. Twenty-four patients in the study underwent GVHD risk assessment per the Mount Sinai Acute GVHD International Consortium scoring algorithm of plasma GVHD biomarkers REG3α and ST2. (A) Treatment response according to GVHD biomarker risk score at enrollment (n = 24). The majority of study participants had high or intermediate risk scores (n = 19); low-risk disease represented the minority of patients under study (n = 5.) Day-28 responses were observed in all risk groups. (B) Assessment of REG3α levels before and after F-652 treatment in 18 patients with paired plasma samples taken at study enrollment and again after completion of F-652 treatment. REG3α levels were significantly greater posttreatment when compared with baseline measurements. (C) Assessment of ST2 levels before and after F-652 treatment in 19 patients with paired plasma samples taken at study enrollment and again after completion of F-652 treatment. ST2 levels showed a mixed response posttreatment when compared with baseline measurements.
IL-22 induces production of innate antimicrobial molecules, including REG family proteins,17,29-31 contributing to gut barrier protection against pathogens.32 We evaluated REG3α plasma concentrations at baseline and following F-652 treatment in a subset of patients with paired pretreatment/posttreatment (day 28) samples (n = 18). Despite the high response rate, circulating REG3α concentrations were significantly increased posttreatment when compared with baseline measurements (Figure 4B). The median concentration of REG3α at baseline was 90 ng/mL, whereas posttherapy it was 257 ng/mL, although REG3α levels did trend down in the 2 patients who had the highest levels pretreatment. In contrast, ST2 concentrations in paired samples (n = 19) demonstrated a mixed response following F-652 treatment, with levels decreasing in 10 patients, increasing in 5 patients, and remaining above the limit of detection in 4 patients (Figure 4C). In addition, although IL-22 has been reported to be associated with inflammation in certain contexts,33-36 we observed no substantial expression changes in a panel of inflammation-associated cytokines using paired samples from before and after F-652 treatment (supplemental Figure 3). Therefore, rhIL-22 treatment did not appear to be associated with increased inflammation in this patient population, and the increase in REG3α levels appeared to be a specific feature of the IL-22 response.
Association between drug PK levels and treatment response
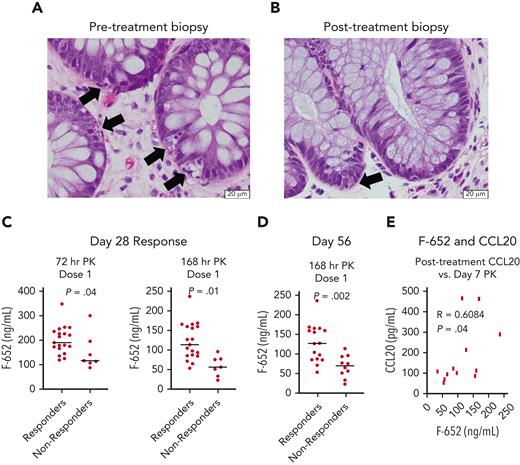
All patients in this study had a baseline GI biopsy performed at the time of enrollment for confirmation of aGVHD histopathology. Two patients with a day-28 CR also underwent repeat endoscopic GI biopsy to evaluate their mucosa after completion of rhIL-22 therapy. Both patients had baseline colonic biopsies showing histologic evidence of aGVHD with marked epithelial apoptotic activity, gland destruction, and gland drop-out. After treatment, their colonic biopsies showed essentially normal glandular architecture with only mild epithelial apoptosis affecting individual cells and without associated gland destruction (Figure 5A-B).
Histology and PK profiles associated with treatment response. (A-B) Representative images of hematoxylin and eosin–stained histopathology performed at the onset of aGVHD and following rhIL-22 treatment with F-652 in a small subset of patients. (A) Pretreatment biopsy: colonic biopsy with crypt injury and extensive epithelial cell apoptosis. Arrows indicate apoptotic cells. (B) Day 28 posttreatment biopsy: colonic mucosa with only rare epithelial apoptotic bodies in basal crypts. Arrow points to a single apoptotic cell. (C) Drug PK levels 3 days and 7 days following the first dose of F-652 were greater in patients who went on to become treatment responders at day 28. (D) Drug PK levels 7 days following the first dose of F-652 were greater in patients who remained treatment responders at day 56. (E) Drug PK levels 7 days following the first dose of F-652 correlated with circulating plasma CCL20 concentrations after completion of F-652 treatment.
Histology and PK profiles associated with treatment response. (A-B) Representative images of hematoxylin and eosin–stained histopathology performed at the onset of aGVHD and following rhIL-22 treatment with F-652 in a small subset of patients. (A) Pretreatment biopsy: colonic biopsy with crypt injury and extensive epithelial cell apoptosis. Arrows indicate apoptotic cells. (B) Day 28 posttreatment biopsy: colonic mucosa with only rare epithelial apoptotic bodies in basal crypts. Arrow points to a single apoptotic cell. (C) Drug PK levels 3 days and 7 days following the first dose of F-652 were greater in patients who went on to become treatment responders at day 28. (D) Drug PK levels 7 days following the first dose of F-652 were greater in patients who remained treatment responders at day 56. (E) Drug PK levels 7 days following the first dose of F-652 correlated with circulating plasma CCL20 concentrations after completion of F-652 treatment.
F-652 PK was assessed according to GVHD treatment response. Levels drawn 1 to 8 hours following the first dose showed no difference in circulating F-652 concentrations between patients who subsequently demonstrated a day-28 treatment response and those who did not (supplemental Figure 4). However, patients who achieved day-28 treatment responses demonstrated significantly higher F-652 concentrations than nonresponders at 72 and 168 hours following dose #1 (Figure 5C). In addition, day-56 treatment response was also associated with higher F-652 serum concentrations 168 hours after dose #1 (Figure 5D).
IL-22BP is a soluble secreted receptor that binds IL-22 and blocks its biologic activity.37,38 However, IL-22BP levels were similar between responders and nonresponders at enrollment and following F-652 treatment (supplemental Figure 5), indicating that inhibition from IL-22BP was unlikely to explain the distinct F-652 PK levels observed between treatment responders and nonresponders. We next investigated associations between F-652 PK levels and expression of CCL20, an IL-22–regulated chemokine.39 F-652 levels measured 7 days after the first dose correlated with posttreatment CCL20 expression (Figure 5E), suggesting that the amount of F-652 detected in circulation may be associated with a measurable in vivo response.
Evaluation of the enteric flora before and after treatment
Recent work has highlighted relationships between the enteric microbiome and clinical GVHD outcomes.40,41 However, little is known about the composition of the flora at the time of GVHD diagnosis and following treatment. IL-22 can impact intestinal health by targeting the epithelium and inducing antimicrobial molecules.16,17,29-31 We thus investigated changes in the stool microbiota from all study participants with evaluable baseline fecal samples at enrollment and after completion of F-652 treatment. Stool specimens were grouped according to day-28 treatment response (responders vs nonresponders).
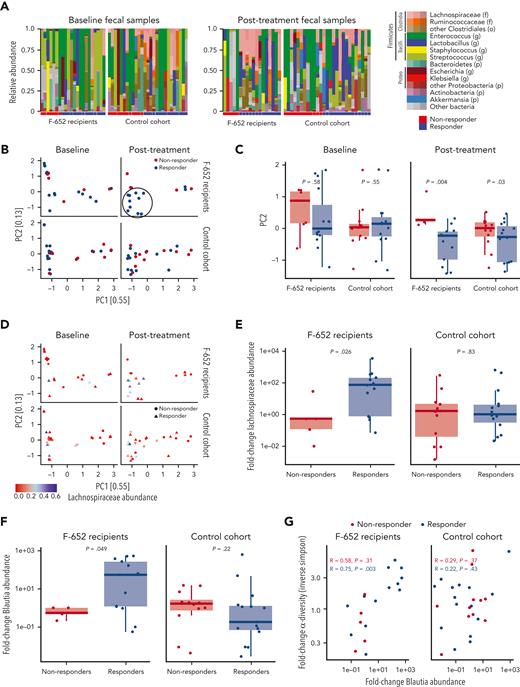
At the time of GVHD presentation, F-652 recipients demonstrated evidence of microbiome dysbiosis, including low abundances of typical commensals and overexpansion of potential pathobionts such as enterococci and streptococci, regardless of subsequent response status (Figure 6A). Following combination therapy with F-652 and corticosteroids, treatment responders appeared to demonstrate an expansion of Lachnospiraceae, a family of commensal Clostridiales anaerobes notable for producing beneficial metabolites such as short-chain fatty acids(Figure 6A).42 Evaluating the global microbiota composition, principal coordinate analysis (PCA) indicated no distinct clustering between treatment-responsive and nonresponding F-652 recipients at GVHD diagnosis (Figure 6B). However, the posttreatment PCA suggested emergence of a distinct cluster of responders among F-652 recipients (Figure 6B), and analysis of β-diversity indicated distinct global compositions between F-652 responders and nonresponders following treatment (Figure 6C), further demonstrating a change in microbial composition in patients treated with F-652 and corticosteroids.
Fecal microbiota analyses before and after treatment of lower GI aGVHD. (A) Overall microbiota composition in recipients of F-652 and corticosteroids (n = 22 patients) or corticosteroids alone (n = 27 patients). At baseline, microbial dysbiosis was present in all cohorts. Posttherapy, fecal samples from F-652 responders indicated an increased proportion of Lachnospiraceae. (B) PCA plots of microbial composition at baseline and following treatment. No distinct clusters can be found pretreatment in recipients of F-652 plus steroids or recipients of steroids alone. Samples clustering together in recipients of F-652 plus steroids following treatment are highlighted in the circle. (C) Comparison of β-diversity, focusing on PC2, prior to treatment and after GI GVHD treatment. (D) Abundance of Lachnospiraceae shown on the PCA plots of responders and nonresponders before and after GVHD treatment, highlighting enrichment of Lachnospiraceae in the previously identified cluster of F-652 responders. (E) Fold-change in Lachnospiraceae abundance following GVHD treatment, indicating Lachnospiraceae expansion in F-652 responders. (F) Fold-change in Blautia abundance following GVHD treatment, indicating Blautia expansion in F-652 responders. (G) Comparison of changes in Blautia abundance and overall microbial diversity indicates a correlation between the 2 parameters for GVHD patients responding to treatment with F-652 and steroids but not for patients treated with steroids alone.
Fecal microbiota analyses before and after treatment of lower GI aGVHD. (A) Overall microbiota composition in recipients of F-652 and corticosteroids (n = 22 patients) or corticosteroids alone (n = 27 patients). At baseline, microbial dysbiosis was present in all cohorts. Posttherapy, fecal samples from F-652 responders indicated an increased proportion of Lachnospiraceae. (B) PCA plots of microbial composition at baseline and following treatment. No distinct clusters can be found pretreatment in recipients of F-652 plus steroids or recipients of steroids alone. Samples clustering together in recipients of F-652 plus steroids following treatment are highlighted in the circle. (C) Comparison of β-diversity, focusing on PC2, prior to treatment and after GI GVHD treatment. (D) Abundance of Lachnospiraceae shown on the PCA plots of responders and nonresponders before and after GVHD treatment, highlighting enrichment of Lachnospiraceae in the previously identified cluster of F-652 responders. (E) Fold-change in Lachnospiraceae abundance following GVHD treatment, indicating Lachnospiraceae expansion in F-652 responders. (F) Fold-change in Blautia abundance following GVHD treatment, indicating Blautia expansion in F-652 responders. (G) Comparison of changes in Blautia abundance and overall microbial diversity indicates a correlation between the 2 parameters for GVHD patients responding to treatment with F-652 and steroids but not for patients treated with steroids alone.
To gain more insight into microbial changes following GVHD treatment, we identified a retrospective cohort of 27 adult patients with lower GI aGVHD treated with systemic corticosteroids (without administration of F-652). This non–IL-22 cohort was closely matched to study participants in terms of age, underlying diagnosis, transplant intensity, and GVHD severity (supplemental Table 3). The most common malignant diagnosis was acute leukemia/myelodysplastic syndrome, the majority of patients received a reduced intensity regimen and an 8/8 HLA–matched unrelated peripheral blood stem cell graft with calcineurin inhibitor–based GVHD prophylaxis, and they had similar GVHD staging (9 stage 1, 4 stage 2, 14 stages 3-4). In addition, day-28 treatment responders (n = 15) and nonresponders (n = 12) were included at a similar proportion to the F-652 study patients. This retrospective cohort was used for evaluation of stool microbiota at GVHD diagnosis and approximately 1 month after treatment. At diagnosis, patients in this non–IL-22 cohort also demonstrated evidence of microbiome dysbiosis with expanded representation of pathobionts (Figure 6A). However, following steroid treatment, no substantial differences were noted in this cohort’s overall microbial composition (Figure 6A), and no distinct clustering or β-diversity changes were identified (Figure 6B-C).
We next assessed whether the differences in microbial composition observed after F-652 administration could be associated with Lachnospiraceae, which appeared more prevalent in treatment responders (Figure 6A). Evaluation of PCA findings based on Lachnospiraceae abundance (Figure 6D) suggested enrichment for high Lachnospiraceae content in the previously identified cluster of responders who received F-652. Furthermore, comparison of samples at GVHD diagnosis and following treatment indicated an increase in relative abundance of Lachnospiraceae among responders treated with F-652 and steroids (Figure 6E). In contrast, no clusters of microbiomes with high Lachnospiraceae abundance were identified in the non–IL-22 cohort (Figure 6D), and no improvement in Lachnospiraceae abundance was found following steroid treatment, even among responders (Figure 6E).
The genus Blautia is a prominent member of the Lachnospiraceae associated with mucosal health, regulatory T cell maintenance, butyrate production, and reduced risk of GVHD-related mortality.21,22,43 In addition to the overall family Lachnospiraceae (Figure 6D), the posttreatment PCA appeared to indicate Blautia-rich microbiomes within the cluster of responding study patients (supplemental Figure 6A), and comparison to pretreatment confirmed Blautia expansion in responders who received F-652 and steroids (Figure 6F). Finally, assessment of microbial α-diversity highlighted the same subset of F-652 responders within the PCA plot (supplemental Figure 6B), and α-diversity was found to correlate with the increased Blautia abundance in responders treated with F-652 and steroids (Figure 6G). Despite the microbial changes observed following F-652 and steroids, evaluation of the non–IL-22 cohort indicated no improvement in Blautia abundance and no correlation with microbial α-diversity after GVHD treatment, even among patients with a successful day-28 response to corticosteroids (Figure 6F-G).
Discussion
Despite pharmacologic advances and growing pathophysiologic insights, GVHD remains a major problem for HCT recipients and a major limitation for the field. Novel therapeutic strategies targeting damaged tissues and promoting their recovery have the potential to complement existing immunosuppressive approaches and improve clinical transplant care. Here, we investigated the potential of an rhIL-22 dimer to enhance recovery of damaged mucosa in newly diagnosed aGVHD and prevent steroid refractory disease. The lower GI day-28 response rate of 70% met the prespecified primary endpoint, and most of the responses remained durable by the day-56 assessment.3,4 Responses were observed across all grades of aGVHD severity, and follow-up posttreatment histopathology in a small subset of responding patients indicated mucosal healing, consistent with observations from IL-22 administration in mouse models.11,12 Although this study was not powered to distinguish statistical differences according to biomarker scores, low, intermediate, and high biomarker risk groups all showed treatment responses.28 Previous work indicated a reduction in circulating aGVHD biomarkers following successful therapy,44 but most F-652 recipients demonstrated an elevation in REG3α levels following treatment, which may represent a beneficial on-target response of IL-22 signaling within the intestinal epithelium leading to increased production of this antimicrobial molecule. Notably, a response rate above 60% was observed even among patients with biomarker-defined high-risk disease. These findings support evaluation in a larger randomized study.
F-652 administration in combination with corticosteroids was well tolerated, as no patients required early treatment discontinuation or dose reduction due to toxicity. The observed AEs were expected for this aGVHD patient population. The most common AEs were cytopenias and electrolyte abnormalities, and most were grade 1 or 2. Xerosis and xeropthalmia were the most common attributable side effects, similar to what was observed in a phase 1 study with F-652 in healthy volunteers.24 Most of these symptoms were mild and transient. Potentially drug-related serious AEs were reported in 9 patients (30%) and consisted of thrombocytopenia and leukopenia. Cytopenias are common complications in patients with aGVHD,45,46 although increased IL-22 levels have been observed in patients with immune thrombocytopenia,47,48 thus warranting further investigation of the cytopenias observed here.
Although IL-22 can promote keratinocyte proliferation and has been associated with skin conditions such as psoriasis,49 we did not identify any cases of psoriasis during the course of this study. We also observed no significant increases in inflammatory cytokine levels following F-652 treatment, and measured cytokine concentrations remained stable compared with baseline assessments. There was no clear risk for epithelial malignancies either, although 1 study patient did develop nonmelanomatous skin cancer, which has been described as a complication observed after allo-HCT.50,51
The observed PK levels were greater than those reported in a previous phase 1 study in healthy human subjects24 but consistent with a more recent study performed in patients with alcoholic hepatitis.52 Notably, we observed an association between higher F-652 trough PK concentrations following the first dose and subsequent day-28 and day-56 treatment responses. This association did not appear to be due to differences in concentrations of the endogenous inhibitor IL-22BP. It is possible that the differing F-652 PK levels between responders and nonresponders reflected accelerated drug clearance in sicker patients, perhaps as a result of protein-losing enteropathy, a known complication of GVHD.53 However, the finding that F-652 trough PK levels correlated with subsequent CCL20 expression suggests the presence of a concentration-dependent in vivo biologic response.
As IL-22 induces epithelial production of innate antimicrobials,29-32 and strong associations have been identified between the enteric microbiome and aGVHD outcomes,21,23,40,54-56 we investigated microbial changes in aGVHD patients treated with F-652 and corticosteroids. A control cohort of newly diagnosed lower GI aGVHD patients treated with systemic corticosteroids in the absence of IL-22 was identified for a comparative analysis of stool microbiota. The 2 cohorts were similar at baseline, showing features of microbial dysbiosis, including an overabundance of the genera Enterococcus and Streptococcus and a lack of commensal anaerobes, features previously observed in transplant patients who go on to develop aGVHD.21,54,56,57 However, patients who responded following F-652 treatment showed a distinct microbial response associated with an expansion of Lachnospiraceae, including an increase in the genus Blautia. Although these microbial associations with treatment response are not evidence of causation, the short chain fatty acid butyrate, which these microbes are known to produce, has been demonstrated experimentally to promote epithelial integrity, intestinal recovery, and overall survival in GVHD mouse models.22 In addition, the increase in Blautia reported here correlated with increased overall microbial diversity, both of which are consistent with improved health of the intestinal microbiota as well as reduced GVHD lethality and TRM.21,58-61 In contrast, standard therapy may possess a limited capacity to improve the enteric microenvironment in patients with GI GVHD. Given the microbial changes observed in F-652 treatment responders, this approach could conceivably synergize with fecal microbiota transplantation and contribute to a normalization of dysbiosis while possibly improving fecal microbiota transplantation safety and containment of transferred pathogens.62
The findings reported in this article highlight the potential of the intestinal microbiome to be developed as a biomarker of treatment responses in GI aGVHD. They also suggest a possible improvement in GVHD-associated dysbiosis after treatment with IL-22 and corticosteroids, supporting further investigation of IL-22 administration as a regenerative immunotherapy associated with a healthier intestinal microbiota profile. As IL-22 does not directly regulate enteric bacteria like antibiotics or even prebiotics, it may represent a class of “peribiotics” able to improve mucosal health and indirectly impact the microbiome. Rather than increasing immunosuppression, IL-22 therapy provides a potential approach for combining immunosuppression with tissue-targeted strategies to promote recovery from immune-mediated damage.
Acknowledgments
The authors thank Jenna Goldberg for her contributions to this study.
This study was sponsored by Evive Biotechnology (Shanghai) Ltd (formerly Generon [Shanghai] Corporation Ltd). The work was also supported in part by a Society Research Grant from the Society of Memorial Sloan Kettering Cancer Center and by the Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748) from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: D.M.P., A.M.A., M.R.M.v.d.B., and A.M.H. designed the study, interpreted the data and wrote the manuscript; R.N., K.S.S., J.N.B., J. Shia, S.G., M.-A.P., A.G., and J.U.P. interpreted the data and wrote the manuscript; J. Slingerland, M.C., and S.D. analyzed and interpreted the data and wrote the manuscript; X.Y., T.T., K.D., J.C., and W.L.D. wrote the manuscript; and G.M., S.F., C.S., L.M., and P.G. collected the data.
Conflict-of-interest disclosure: D.M.P., A.M.A., S.G., M.-A.P., and A.M.H. served as advisory board members for Evive Biotechnology (Shanghai) Ltd (formerly Generon [Shanghai] Corporation Ltd). M.R.M.v.d.B. and A.M.H. hold intellectual property related to IL-22 treatment in GVHD. A.M.H. serves in a volunteer capacity as a member of the Board of Directors of the American Society for Transplantation and Cellular Therapy (ASTCT). J. Shia serves as a consultant for Paige AI. D.M.P. serves as consultant to Kadmon/Sanofi Corporation, CareDx, Incyte and Ceramedix, and receives research funding from Incyte. M.-A.P. reports honoraria from Adicet, Allovir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omeros; has received institutional research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis; and serves in a volunteer capacity as a member of the Board of Directors of the ASTCT and Be The Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Executive Committee. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from, or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaxoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (spouse), Synthekine (spouse), Beigene (spouse), Kite (spouse); has intellectual property licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the Foundation Board of DKMS (a nonprofit organization). The remaining authors declare no competing financial interests.
Correspondence: Doris M. Ponce, Department of Medicine, Memorial Sloan Kettering Cancer Center, Box 68, 545 E 73rd St, New York, NY, 10021; e-mail: ponced@mskcc.org; and Alan Hanash, Department of Medicine and Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: hanasha@mskcc.org.
References
Author notes
Data are available on request from the corresponding authors, Doris M. Ponce and Alan Hanash (ponced@mskcc.org; hanasha@mskcc.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal