Key Points
Hydroxyurea at MTD is associated with a significant and sustained lower malaria incidence among children with sickle cell anemia in Africa.
An absolute neutrophil count below 3.0 × 109/L is an important treatment threshold, below which the malaria incidence is reduced.
Abstract
Realizing Effectiveness Across Continents with Hydroxyurea (REACH, NCT01966731) provides hydroxyurea at maximum tolerated dose (MTD) for children with sickle cell anemia (SCA) in sub-Saharan Africa. Beyond reducing SCA-related clinical events, documented treatment benefits include ∼50% reduction in malaria incidence. To identify associations and propose mechanisms by which hydroxyurea could be associated with lower malaria rates, infections were recorded across all clinical sites (Angola, Democratic Republic of Congo, Kenya, and Uganda). Hazard ratios (HR) with 95% confidence intervals (CIs) for baseline demographics, and time-varying laboratory and clinical parameters were estimated in a modified Cox gap-time model for repeated events. Over 3387 patient-years of hydroxyurea treatment, 717 clinical malaria episodes occurred in 336 of 606 study participants; over half were confirmed by blood smear and/or rapid diagnostic testing with 97.8% Plasmodium falciparum. In univariate analysis limited to 4 confirmed infections per child, malaria risk was significantly associated with absolute neutrophil count (ANC), splenomegaly, hemoglobin, and achieving MTD; age, malaria season, MTD dose, fetal hemoglobin, α-thalassemia, and glucose-6-phosphate dehydrogenase deficiency had no effect. In multivariable regression of confirmed infections, ANC was significant (HR, 1.37 per doubled value; 95% CI, 1.10-1.70; P = .0052), and ANC values <3.0 × 109/L were associated with lower malaria incidence. Compared with nonpalpable spleen, 1- to 4-cm splenomegaly also was associated with higher malaria risk (HR, 2.01; 95% CI, 1.41-2.85; P = .0001). Hydroxyurea at MTD is associated with lower malaria incidence in SCA through incompletely defined mechanisms, but treatment-associated mild myelosuppression with ANC <3.0 × 109/L is salutary. Splenomegaly is an unexplained risk factor for malaria infections among children with SCA in Africa.
Introduction
Realizing Effectiveness Across Continents with Hydroxyurea (REACH; ClinicalTrials.govNCT01966731; Pan-African clinical trials registry PACTR202105884908295) is a phase 1/2 prospective multicenter trial of hydroxyurea for sickle cell anemia (SCA). The REACH trial is designed to determine the safety, feasibility, and benefits of hydroxyurea at maximum tolerated dose (MTD) for children with SCA living in sub-Saharan Africa.1 Children with SCA at 4 African clinical sites (Luanda, Angola; Kinshasa, Democratic Republic of Congo; Kilifi, Kenya; and Mbale, Uganda) were treated with hydroxyurea at a fixed dose (15-20 mg/kg/d) for 6 months, followed by dose escalation to MTD. A total of 635 children with SCA were enrolled, and after a screening period, 606 children from ages 1 to 10 years–initiated hydroxyurea treatment.2
The primary results of REACH documented both the safety and feasibility of hydroxyurea treatment in this patient population as well as expected benefits for both laboratory and clinical complications of SCA. Specifically, laboratory benefits included higher hemoglobin (Hb) concentration, mean corpuscular volume (MCV), and fetal hemoglobin (HbF), as well as lower white blood cell (WBC) count, absolute neutrophil count (ANC), absolute reticulocyte count, and platelet count.3 Clinical benefits included predicted reductions in the incidence rate ratio (IRR) of acute clinical SCA-related complications, such as vaso-occlusive pain and acute chest syndrome, but unexpected benefits were also documented. Over the first 30 months of treatment, a statistically significant ∼50% reduction in malaria infections (IRR, 0.49; 95% confidence interval [CI], 0.37-0.66; P < .001) was observed compared with rates during the screening period, along with even greater reductions in blood transfusions (IRR, 0.33; 95% CI, 0.23-0.47; P < .001) and death (IRR, 0.30; 95% CI, 0.10-0.88; P = .029).3
Despite concerns about an increased risk of malaria in association with hydroxyurea treatment, the observed ∼50% decrease in the incidence of malaria was among the most intriguing and unexpected observations in the initial REACH trial results and remains incompletely understood. Notably, the decreased rate occurred at all 4 clinical sites across Africa, was most pronounced after achieving the MTD dose, and was sustained over time.3 We therefore performed a longitudinal analysis of all malaria events in the REACH study to date, focusing on confirmed infections, to identify associations between hydroxyurea treatment and reduced malaria infection rates in African children with SCA.
Methods
Trial design
The REACH trial design has been previously published.1 Briefly, children with SCA at 4 clinical sites in sub-Saharan Africa, from 1 to 10 years of age at the time of enrollment, were recruited. After a 2-month screening period, the children were initially treated with open-label oral hydroxyurea capsules at a fixed dose of 15 to 20 mg/kg per day for 6 months, followed by dose escalation to MTD. The primary study end point was the percentage of children with hematological dose-limiting toxicities during the first 3 months of treatment, whereas secondary study end points included laboratory and clinical benefits such as vaso-occlusive events, infections, and transfusions.
Hydroxyurea treatment
A total of 635 children were enrolled into REACH, ranging from 151 in Angola to 161 in Kenya. After completing the screening phase, 606 children commenced hydroxyurea treatment at 17.5 ± 1.8 mg/kg per day for 6 months, followed by stepwise dose escalation based on serial blood counts to achieve MTD, defined as mild myelosuppression with an ANC of 2.0 × 109/L to 4.0 × 109/L.1 A total of 519 REACH study participants (86%) achieved MTD at an average (mean ± standard deviation) hydroxyurea dose of 22.5 ± 4.9 mg/kg/d (range, 18.9 ± 4.2 to 25.3 ± 4.8 mg/kg/d) after a median of 11 months of study treatment, with significant increases in Hb, MCV, and HbF.3 Subsequently, the participants have had further dose optimization with adjustments for weight gain and laboratory values.
Data collection
Scheduled visits, which included a pertinent interval medical history and physical examination, occurred monthly during the first year of treatment, then every 2 months, and eventually at 3-month intervals. At each visit, the spleen size was assessed by palpation by an experienced physician, with recording of the maximal size (in cm) below the left costal margin at the midaxillary and/or midclavicular line. Complete blood counts were obtained along with reticulocytes quantified by automated measurement or manual counting. HbF was measured every 3 to 6 months in the first year and annually thereafter by either capillary electrophoresis or high-performance liquid chromatography. Genetic modifiers included the 3.7 kb α-globin gene deletion (−α3.7) for α-thalassemia and the A− variant for glucose-6-phosphate dehydrogenase (G6PD A−) deficiency, both identified by DNA-based methods as previously described.4 Medical history, physical examination, and laboratory and clinical adverse events, grade 2 severity and above, were recorded into a REDCap database at all scheduled and unscheduled visits, with both on-site and remote data monitoring performed by the US-based Data Management Center.1
Malaria infections
At each scheduled or unscheduled visit, site personnel asked about signs and symptoms of malaria since the previous encounter, including infections treated in primary or secondary health care facilities. All diagnoses of malaria based either on past or current symptoms are hereafter referred to as “clinical malaria infections” with sites recording medications, transfusions, medical courses, and laboratory data. To confirm the diagnosis of malaria in study participants who presented to the REACH clinical sites with signs and symptoms such as fever, headache, or pallor, thick blood smears were scored by visual documentation of malarial parasite density per 200 or 500 WBC and recorded as parasites per 100 WBC or per microliter blood. In some cases, malaria rapid diagnostic test (RDT) results were also performed at the REACH clinical sites, especially if the participant had recently been treated at a health care facility before the visit. These diagnoses of malaria with a positive blood smear and/or RDT are hereafter referred to as “confirmed malaria infections” in the statistical analyses. During sick visits, blood was also collected on Whatman Flinders Technology Associates cards and analyzed later at Cincinnati Children’s Hospital for the presence of plasmodium DNA by real-time polymerase chain reaction, using published methods for malaria density and speciation.5
Statistical analyses
Baseline demographics, clinical, and laboratory variables were summarized for the REACH cohort, first for the entire set of clinical malaria infections and then for the confirmed malaria infections (blood smear and/or RDT). The analysis of malaria episodes used the Prentice-Williams-Peterson extension of the Cox model for times between incident malaria infections,6,7 limited to the first 4 episodes per child to avoid associations being inappropriately influenced by 13 children with 5 or more confirmed episodes. The functional form for the relationships between the risk of confirmed malaria and 3 lab values (ANC, MCV, and HbF) was assessed in the Cox model by fitting models with penalized cubic splines and linear terms for these variables and comparing the model fits with a Wald test; linear terms were adequate for HbF and MCV but not for ANC (supplemental Data for details, available on the Blood website).
Univariate and multivariable models, with stratification by site, were fitted for fixed baseline variables age, sex, and attainment of MTD; site-specific malaria season (low, moderate, and high); most recent laboratory values HbF, log2(ANC), and MCV (all lagged by at least 15 days and at least 15 days after a previous malaria infection); and spleen status (4 categories: not palpable, palpable 1-4 cm, palpable ≥5 cm, or splenectomy), which was lagged by at least 30 days. Lagging was used to ensure that the time-updated predictor variable was assessed well before the episode of malaria and therefore not affected by any malaria infection–related changes. α-Thalassemia was analyzed as the normal 4-globin gene complement with no gene deletions (αα/αα) vs 1-gene deletion (−α3.7/αα) or 2-gene deletion (−α3.7/−α3.7). The G6PD A− genotype was analyzed as either normal or hemizygous affected males and as normal, heterozygous carrier, or homozygous affected females.
Hb was assessed in univariate analyses, but because low values can persist after a malaria infection, we excluded Hb from our main analyses. The fitted spline function for ANC was closely approximated by using the log-transformed value of ANC. For ease of presentation and interpretation, the logarithm of ANC to base 2 (doubling the value) was used in the model. The hazard ratio (HR) was calculated for each predictor, along with 95% CI and P value for a test of no association.
Ethical review
The REACH trial received all appropriate approvals and annual renewals at all 4 clinical sites, including local ethics boards and national regulatory agencies, as well as Cincinnati Children’s Hospital Medical Center, which serves as the institutional review board of record. All families signed informed consent for participation and treatment. The REACH data management center compiled the data for analysis by study biostatisticians (G.T. and A.L.), but all investigators had access to the trial results and approved the final version of the manuscript.
Results
Between September 2014 and January 2022, a total of 717 clinical malaria infections were recorded in the REACH cohort over 3387 patient-years of hydroxyurea treatment. This included 336 children (55.4%) with at least 1 clinical infection, 171 (28.2%) with at least 2 infections, 88 (14.7%) with at least 3 infections, and 58 (9.6%) with at least 4 infections. The proportion of children with no recorded malaria infections varied by clinical site, ranging from 27% in the Democratic Republic of Congo to 70% in Angola, with intermediate values recorded for Kenya and Uganda. The proportion of children with at least 3 malaria infections ranged from 1% in Angola to 28% in Democratic Republic of Congo.
There were 7 children (5 females, 2 males; age range, 5-13 years) whose deaths were attributed to malaria during the treatment period, typically presenting with an acute febrile illness, severe anemia, and a positive blood smear for malaria parasites, and receiving initial management in rural settings outside of the main REACH clinical sites. These 7 deaths represent 1.2% of the study participants and 1.0% of the total number of recorded clinical malaria infections. Four of the children were prescribed chemoprophylaxis at the time of their illness; the clinical management of several children who died was hindered by COVID-related travel restrictions and a lack of available blood. None of the malaria-associated deaths occurred in children with a previous surgical splenectomy.
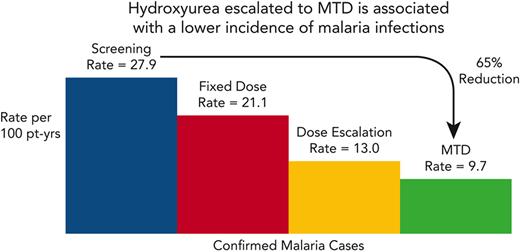
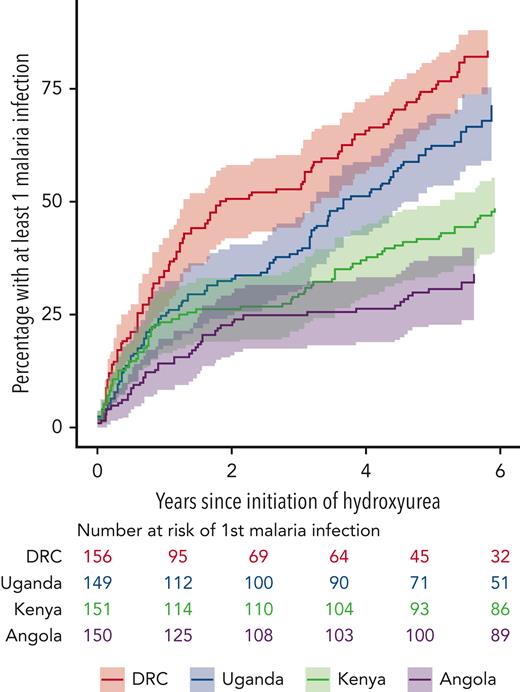
REACH study participants in Angola and the Democratic Republic of Congo were not prescribed malaria prophylaxis, whereas children in Kenya were prescribed oral proguanil 100 mg once daily, and those in Uganda received monthly sulfadoxine/pyrimethamine, following the national guidelines for each country. Figure 1 shows the cumulative incidence of the first clinical malaria infection per child over time since initiation of hydroxyurea. The Democratic Republic of Congo had the highest number of on-treatment clinical malaria cases (N = 283), followed by Uganda (N = 195), Kenya (N = 193), and Angola (N = 56). Although we do not have a clear explanation for this variation among the 4 clinical sites, a reduction in malaria incidence was observed during treatment at all 4 sites in the REACH trial. Figure 2 illustrates the overall incidence of confirmed malaria infections in 6-month intervals, extending to 6 years of hydroxyurea treatment. For the entire study cohort, the rate of confirmed malaria infections was reduced from 27.9 per 100 patient-years during screening to 21.1 on fixed-dose hydroxyurea, which then fell further to 13.0 during dose escalation and 9.7 at hydroxyurea MTD; overall, the malaria incidence was 11.6 per 100 patient-years averaging all phases of hydroxyurea treatment.
Clinical malaria infections in the REACH cohort. The figure shows cumulative incidence of the first episode of malaria by clinical site, along with the numbers of children at each site still being followed and free of a malaria infection.
Clinical malaria infections in the REACH cohort. The figure shows cumulative incidence of the first episode of malaria by clinical site, along with the numbers of children at each site still being followed and free of a malaria infection.
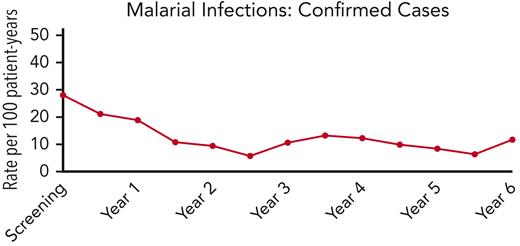
Malaria infections over time in the REACH cohort. The incidence of confirmed malaria infections is plotted against time, and illustrates a reduction from 27.9 infections per 100 patient-years during screening to an average of 11.6 infections per 100 patient-years after starting hydroxyurea treatment, with the lower rates sustained for 6 years.
Malaria infections over time in the REACH cohort. The incidence of confirmed malaria infections is plotted against time, and illustrates a reduction from 27.9 infections per 100 patient-years during screening to an average of 11.6 infections per 100 patient-years after starting hydroxyurea treatment, with the lower rates sustained for 6 years.
After limiting to a maximum of 4 episodes per child, there were a total of 653 clinical malaria infections recorded on hydroxyurea treatment, of which 346 (53%) were confirmed malaria infections used in the primary analysis. Subsequent diagnostic analysis of these confirmed cases was performed by central PCR-based analysis of Whatman Flinders Technology Associates cards collected on the same day as the malaria diagnosis; plasmodium DNA was identified in 182 of the 191 samples (95.3%) and Plasmodium falciparum DNA was detected in 178 of the 182 (97.8%) malaria positive cards.
Enrollment demographics, clinical, and laboratory data were first compared between study participants who ever developed malaria infections and those who never developed malaria on hydroxyurea. Baseline parameters including age, sex, height, weight, key laboratory values (Hb, ANC, MCV, HbF), spleen presence or size, G6PD genotype in males or females, or α-thalassemia status were not associated with the subsequent development of malaria during the REACH treatment period; however, a history of previous malaria infections at the time of enrollment was significantly higher in the children who then developed malaria on hydroxyurea (Table 1).
Enrollment demographics and risk of developing confirmed malaria infection in REACH
| Enrollment parameter . | Confirmed malaria infections (N = 346) . | ||
|---|---|---|---|
| Malaria . | No malaria . | P value . | |
| Number of study participants | 218 | 388 | -- |
| Age (y), mean (SD) | 5.5 (2.3) | 5.4 (2.4) | .55 |
| Sex (male, %) | 117 (53.7) | 193 (49.7) | .40 |
| Pre-enrollment malaria, n (%) | 199 (91.3) | 318 (82.0) | .0019 |
| Height (cm), mean (SD) | 107.6 (14.0) | 107.7 (15.2) | .97 |
| Weight (kg), mean (SD) | 17.3 (4.3) | 17.3 (4.8) | .92 |
| Hb (g/dL), mean (SD) | 7.2 (1.2) | 7.4 (1.0) | .029 |
| MCV (fL), mean (SD) | 76.9 (9.4) | 76.2 (8.7) | .32 |
| ANC (×109/L), med (IQR) | 6.4 (5.0-8.2) | 6.1 (4.8-8.1) | .38 |
| HbF (%), mean (SD) | 10.0 (6.0) | 10.4 (6.5) | .42 |
| Spleen status | .52 | ||
| Nonpalpable, n (%) | 173 (80.8) | 312 (83.9) | |
| Palpable (1-4 cm), n (%) | 18 (8.4) | 32 (8.6) | |
| Palpable (≥5 cm), n (%) | 19 (8.9) | 25 (6.7) | |
| Splenectomy, n (%) | 4 (1.9) | 3 (0.8) | |
| α-thalassemia genotypes | .15 | ||
| 2 gene deletion (−α3.7/−α3.7) | 21 (9.6) | 43 (11.1) | |
| 1 gene deletion (−α3.7/αα) | 95 (43.6) | 195 (50.3) | |
| None (αα/αα) | 102 (46.8) | 150 (38.7) | |
| G6PD genotypes | .30 | ||
| Male unaffected | 99 (84.6) | 150 (77.7) | |
| Male hemizygous | 18 (15.4) | 43 (22.3) | |
| Female unaffected | 65 (64.4) | 124 (63.6) | |
| Female heterozygous | 32 (31.7) | 68 (34.9) | |
| Female homozygous | 4 (4.0) | 3 (1.5) | |
| Enrollment parameter . | Confirmed malaria infections (N = 346) . | ||
|---|---|---|---|
| Malaria . | No malaria . | P value . | |
| Number of study participants | 218 | 388 | -- |
| Age (y), mean (SD) | 5.5 (2.3) | 5.4 (2.4) | .55 |
| Sex (male, %) | 117 (53.7) | 193 (49.7) | .40 |
| Pre-enrollment malaria, n (%) | 199 (91.3) | 318 (82.0) | .0019 |
| Height (cm), mean (SD) | 107.6 (14.0) | 107.7 (15.2) | .97 |
| Weight (kg), mean (SD) | 17.3 (4.3) | 17.3 (4.8) | .92 |
| Hb (g/dL), mean (SD) | 7.2 (1.2) | 7.4 (1.0) | .029 |
| MCV (fL), mean (SD) | 76.9 (9.4) | 76.2 (8.7) | .32 |
| ANC (×109/L), med (IQR) | 6.4 (5.0-8.2) | 6.1 (4.8-8.1) | .38 |
| HbF (%), mean (SD) | 10.0 (6.0) | 10.4 (6.5) | .42 |
| Spleen status | .52 | ||
| Nonpalpable, n (%) | 173 (80.8) | 312 (83.9) | |
| Palpable (1-4 cm), n (%) | 18 (8.4) | 32 (8.6) | |
| Palpable (≥5 cm), n (%) | 19 (8.9) | 25 (6.7) | |
| Splenectomy, n (%) | 4 (1.9) | 3 (0.8) | |
| α-thalassemia genotypes | .15 | ||
| 2 gene deletion (−α3.7/−α3.7) | 21 (9.6) | 43 (11.1) | |
| 1 gene deletion (−α3.7/αα) | 95 (43.6) | 195 (50.3) | |
| None (αα/αα) | 102 (46.8) | 150 (38.7) | |
| G6PD genotypes | .30 | ||
| Male unaffected | 99 (84.6) | 150 (77.7) | |
| Male hemizygous | 18 (15.4) | 43 (22.3) | |
| Female unaffected | 65 (64.4) | 124 (63.6) | |
| Female heterozygous | 32 (31.7) | 68 (34.9) | |
| Female homozygous | 4 (4.0) | 3 (1.5) | |
SD, standard deviation; IQR, 25% to 75% interquartile range.
The results from univariate analyses of confirmed malaria infections, using lagged time-dependent variables for the most recent laboratory values and clinical status, identified significant associations for achieving MTD, higher ANC, lower Hb, 1-4 cm palpable spleen, and 2-gene deletional α-thalassemia (Table 2). No significant associations were identified between malaria infections and age, sex, HbF, MCV, malaria season, or G6PD genotype. Multivariable regression analysis was then performed (Table 3) using a model incorporating HbF but not MTD or Hb. For confirmed malaria infections, only 2 variables remained significant: higher ANC (HR, 1.37; 95% CI, 1.10-1.70; P = .0052) and 1-4 cm palpable splenomegaly (HR, 2.01; 95% CI, 1.41-2.85; P = .0001).
Univariate HR and 95% CI for malaria infection (limited to 4 episodes) for confirmed malaria infections
| Parameter . | Confirmed malaria infections (N = 346) . | ||
|---|---|---|---|
| HR . | 95% CI . | P value . | |
| Age (per y) | 1.01 | 0.96-1.05 | .70 |
| Sex (male vs female) | 1.06 | 0.85-1.33 | .58 |
| MTD reached | 0.63 | 0.49-0.81 | .0004 |
| HbF ratio (per 10% increase) | 0.94 | 0.83-1.05 | .26 |
| Hb (per 1 g/dL increase) | 0.78 | 0.73-0.85 | <.0001 |
| ANC (per doubling) | 1.30 | 1.05-1.61 | .017 |
| MCV (per 10 fL increase) | 1.09 | 0.99-1.19 | .08 |
| Malaria season (vs low) | |||
| High | 1.11 | 0.76-1.62 | .58 |
| Moderate | 1.02 | 0.79-1.31 | .90 |
| Spleen status (vs nonpalpable) | |||
| Palpable (1-4 cm) | 2.05 | 1.44-2.94 | .0001 |
| Palpable (≥5 cm) | 1.45 | 0.91-2.29 | .12 |
| Splenectomy | 1.40 | 0.82-2.39 | .21 |
| α-Thalassemia (vs 4 genes) | |||
| 3 genes (−α3.7/αα) | 0.88 | 0.70-1.12 | .30 |
| 2 genes (−α3.7/−α3.7) | 0.69 | 0.49-0.96 | .026 |
| G6PD genotype (vs normal) | |||
| Male (hemizygous A-) | 1.09 | 0.76-1.56 | .64 |
| Female (heterozygous A) | 1.04 | 0.67-1.61 | .86 |
| Parameter . | Confirmed malaria infections (N = 346) . | ||
|---|---|---|---|
| HR . | 95% CI . | P value . | |
| Age (per y) | 1.01 | 0.96-1.05 | .70 |
| Sex (male vs female) | 1.06 | 0.85-1.33 | .58 |
| MTD reached | 0.63 | 0.49-0.81 | .0004 |
| HbF ratio (per 10% increase) | 0.94 | 0.83-1.05 | .26 |
| Hb (per 1 g/dL increase) | 0.78 | 0.73-0.85 | <.0001 |
| ANC (per doubling) | 1.30 | 1.05-1.61 | .017 |
| MCV (per 10 fL increase) | 1.09 | 0.99-1.19 | .08 |
| Malaria season (vs low) | |||
| High | 1.11 | 0.76-1.62 | .58 |
| Moderate | 1.02 | 0.79-1.31 | .90 |
| Spleen status (vs nonpalpable) | |||
| Palpable (1-4 cm) | 2.05 | 1.44-2.94 | .0001 |
| Palpable (≥5 cm) | 1.45 | 0.91-2.29 | .12 |
| Splenectomy | 1.40 | 0.82-2.39 | .21 |
| α-Thalassemia (vs 4 genes) | |||
| 3 genes (−α3.7/αα) | 0.88 | 0.70-1.12 | .30 |
| 2 genes (−α3.7/−α3.7) | 0.69 | 0.49-0.96 | .026 |
| G6PD genotype (vs normal) | |||
| Male (hemizygous A-) | 1.09 | 0.76-1.56 | .64 |
| Female (heterozygous A) | 1.04 | 0.67-1.61 | .86 |
For HR calculations, the reference comparators were female sex, low malaria season, nonpalpable spleen, normal (4-gene) α-globin, and normal G6PD genotype of the same sex.
Multivariable HR and 95% CI for malaria infection (limited to 4 episodes) for confirmed malaria infections
| Parameter . | Confirmed malaria infections (N = 346) . | ||
|---|---|---|---|
| HR . | 95% CI . | P value . | |
| Age (per y) | 1.02 | 0.97-1.07 | .47 |
| HbF ratio (per 10% increase) | 1.00 | 0.87-1.13 | .94 |
| ANC (per doubling of the value) | 1.37 | 1.10-1.70 | .0052 |
| MCV (per 10 fL increase) | 1.11 | 0.99-1.26 | .78 |
| Malaria season (vs low) | |||
| High | 1.05 | 0.82-1.35 | .69 |
| Moderate | 0.77 | 0.55-1.07 | .12 |
| Spleen status (vs nonpalpable) | |||
| Palpable (1-4 cm) | 2.01 | 1.41-2.85 | .0001 |
| Palpable (≥5 cm) | 1.32 | 0.85-2.05 | .22 |
| Splenectomy | 1.13 | 0.74-1.71 | .58 |
| Parameter . | Confirmed malaria infections (N = 346) . | ||
|---|---|---|---|
| HR . | 95% CI . | P value . | |
| Age (per y) | 1.02 | 0.97-1.07 | .47 |
| HbF ratio (per 10% increase) | 1.00 | 0.87-1.13 | .94 |
| ANC (per doubling of the value) | 1.37 | 1.10-1.70 | .0052 |
| MCV (per 10 fL increase) | 1.11 | 0.99-1.26 | .78 |
| Malaria season (vs low) | |||
| High | 1.05 | 0.82-1.35 | .69 |
| Moderate | 0.77 | 0.55-1.07 | .12 |
| Spleen status (vs nonpalpable) | |||
| Palpable (1-4 cm) | 2.01 | 1.41-2.85 | .0001 |
| Palpable (≥5 cm) | 1.32 | 0.85-2.05 | .22 |
| Splenectomy | 1.13 | 0.74-1.71 | .58 |
For HR calculations, the reference comparators were female sex, low malaria season, and a nonpalpable spleen.
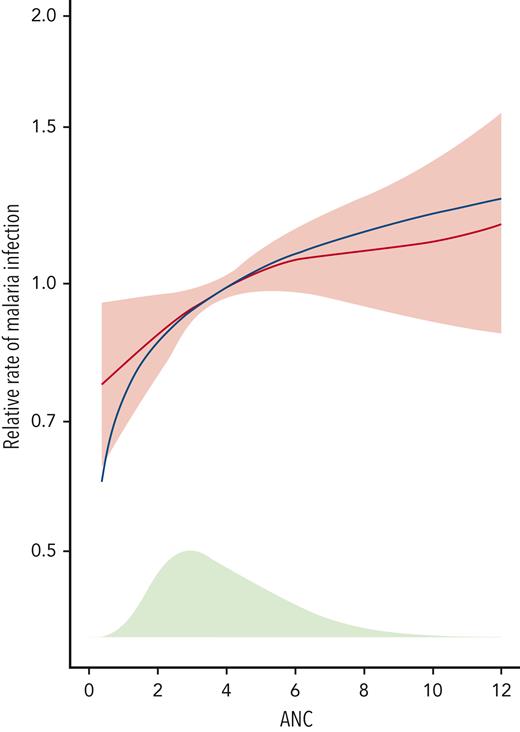
Recognizing that malaria was reduced overall across the whole REACH treatment cohort but that ANC was a key variable associated with the risk of malaria, further analysis was performed. As illustrated in Figure 3, there was a nonlinear correlation with a greater decrease in risk (HR < 1.0) at lower ANC values compared with higher ANC values, with a sharp decrease in malaria risk observed at an ANC below 3.0 × 109/L. Other variables, including HbF and MCV, did not have a similar threshold value (data not shown).
Malaria risk and ANC in the REACH cohort. Using results from the multivariable model for confirmed malaria infections in Table 3, the estimated hazard ratio (HR) and 95% confidence intervals from the fit with log2(ANC) are shown in red. The figure is scaled so that a study participant with the mean ANC value at 24 months has an HR of 1. The blue line shows the fit for the more flexible 4-degree of freedom penalized spline for ANC, to illustrate that the fit using log2(ANC) is a good approximation. The green shaded area at the bottom of the plot shows the distribution of the ANC at 24 months. There is a non-linear correlation with greater decrease in risk (HR<1.00) at lower ANC values, compared to higher values, and a sharper decrease in malaria risk at ANC values below 3.0 x 109/L.
Malaria risk and ANC in the REACH cohort. Using results from the multivariable model for confirmed malaria infections in Table 3, the estimated hazard ratio (HR) and 95% confidence intervals from the fit with log2(ANC) are shown in red. The figure is scaled so that a study participant with the mean ANC value at 24 months has an HR of 1. The blue line shows the fit for the more flexible 4-degree of freedom penalized spline for ANC, to illustrate that the fit using log2(ANC) is a good approximation. The green shaded area at the bottom of the plot shows the distribution of the ANC at 24 months. There is a non-linear correlation with greater decrease in risk (HR<1.00) at lower ANC values, compared to higher values, and a sharper decrease in malaria risk at ANC values below 3.0 x 109/L.
Discussion
Malaria infections are a major cause of morbidity and mortality for children living in Africa, but current efforts that provide insecticide-treated bed nets and periodic spraying, as well as chemoprophylaxis and new vaccines, are lowering the burden in some locations.8,9 Although the incidence of infection in SCA may not actually be increased,10 the risk for death from malaria has historically been considered higher than in other children because of underlying severe anemia and poor splenic filtrative function.11 However, more recent data suggest that even for children with SCA, prompt diagnosis and management of malaria can be life-saving.12 In REACH, children with SCA at all 4 clinical sites frequently had malaria infections (Figure 1), but with an overall low mortality rate of only 1% of recorded infections. Several of these deaths occurred when children were initially managed in lower-level health care facilities before transfer to the REACH clinical sites.
There are few published data regarding the clinical effects of hydroxyurea on malaria infections. The NOHARM trial was a double-blind, placebo-controlled trial designed to test the rate of malaria in children with SCA on hydroxyurea vs placebo, with ∼100 patient-years in each study arm, but the infection rate was relatively low in Kampala, Uganda during the 12-month treatment period.13 NOHARM documented ∼30% reduction in malaria for children on hydroxyurea compared with placebo, but this difference was not statistically significant because of low absolute numbers. In REACH, with several hundred cases of malaria and several thousand patient-years of hydroxyurea treatment, we were able to perform strong statistical analyses and derive conclusions that could improve clinical care and outcomes for this vulnerable patient population.
While receiving hydroxyurea, REACH study participants had an unexpected and significant reduction in malaria infections, compared with the pretreatment screening period. In our initial results paper, the incidence of clinical malaria infections fell from 46.9 per 100 patient-years during screening to 22.9 per 100 patient-years of treatment, with an IRR of 0.49 (95% CI, 0.37-0.66).3 Now with over 3300 patient-years of hydroxyurea treatment, the incidence of confirmed malaria on hydroxyurea treatment is 11.2 infections per 100 patient-years, documenting a substantial and sustained >50% reduction in the infection incidence rate (Figure 2). Because the REACH study participants were 5.5 years old at the time of enrollment and the decrease in the malaria rate was observed in the first 6 to 12 months of treatment, we do not believe that the decline is based on increasing age. Further, because enrollment occurred over a 2-year period and covered all calendar months and both rainy/dry seasons, we believe that our cumulative screening period (110 patient-years) provides an accurate pretreatment rate before initiating hydroxyurea. Although we cannot definitively exclude a longitudinal or reporting bias, the observed reductions in malaria infections are likely accurate and associated with the hydroxyurea treatment.
The overall malaria rate observed in the REACH cohort may seem high, given the local practices to control infections using chemoprophylaxis and bed nets. However, the 4 clinical sites had a variable approach to malaria prevention, with chemoprophylaxis part of the national guidelines in Kenya and Uganda but not in Angola or the Democratic Republic of Congo. Moreover, even with good adherence, the use of antimalarial prophylaxis is well documented to afford only incomplete protection against malaria,14,15 likely because of low antiparasitic effectiveness and increasing rates of drug resistance.
To investigate the protective effects of hydroxyurea against malaria infections, we analyzed a variety of baseline demographics, laboratory, clinical, and genetic factors; none of them were consistently different between children who developed malaria and those who did not, except for having previous malaria at the time of enrollment, which could simply represent exposure risk. Because the presence of G6PD A− deficiency is usually associated with a lower risk of malaria,16 especially among heterozygous females,17 it was notable that the G6PD genotype was not protective in the REACH cohort. However, the protective effects of G6PD A− deficiency are complex and may primarily reduce the risk of cerebral malaria.18,19 Similarly, α-thalassemia trait is known to protect against malaria20,21 but demonstrated no consistent protective effect in our analyses. Although these observations could reflect our exclusively African cohort or the sample size, it is more likely that these inherited traits afford protection against malaria infections only in otherwise normal children; in the setting of SCA, negative epistasis can occur and reduce their individual protective effects.22
Using univariate and then multivariable analysis in confirmed malaria infections, we identified the circulating neutrophil count and spleen status as the 2 most important parameters affecting malaria risk. Neutrophils are important in malaria, forming an initial immune response characterized by phagocytosis of circulating parasites with the release of abundant reactive oxygen species and neutrophil extracellular traps, but potentially later with damaging effects on the endothelium and deleterious effects on overall bacterial defense.23,24 In REACH, despite hydroxyurea being associated with an overall significant reduction in malaria infections, ANC was an additional important parameter with ∼30% increased risk for each doubling of the value (Table 3). There was a nonlinear decline in the HR at lower ANC values, with a sharp decline in the malaria risk below 3.0 × 109/L (Figure 3). This observation has relevance for hydroxyurea treatment because dose escalation to MTD is designed to cause mild myelosuppression with an optimal ANC of 1.0 × 109/L to 3.0 × 109/L.25 Higher hydroxyurea doses can therefore maximize HbF induction and are associated with a lower malaria incidence. Whether lower ANC values have direct effects to decrease malaria risk through reduced adhesion, inflammation, and endothelial activation, or simply serve as a proxy for an optimal hydroxyurea dose that has other antimalarial benefits remains to be determined.
Spleen status was also an important predictor of malaria risk, with <5 cm (1-4 cm) palpable splenomegaly associated with an increased incidence (Tables 2 and 3). Because the spleen is the main location of blood filtration, it serves as an important site of parasite clearance, and splenectomy is recognized as a risk factor for severe malaria.26,27 In SCA, the spleen is damaged early in life, but hydroxyurea treatment may preserve or even recover filtrative function.28,29 In malaria-endemic regions, palpable splenomegaly is normally considered a sign of acute infection but not necessarily a risk factor for subsequent infection. One study documented better clinical outcomes from severe malaria in children with a larger spleen volume, possibly related to better filtrative function.30 Our observations suggest that mild splenomegaly (1-4 cm) could also reflect preserved spleen parenchyma, restored splenic function, or potentially incomplete malaria treatment. We further speculate that a large spleen could potentially serve as a reservoir or shelter for P falciparum malaria parasites, although we present no direct evidence for this. However, this concept is consistent with ex vivo studies31,32 and a small observational trial in Nigeria, which reported that 10 of 13 young patients with SCA had improvement of splenomegaly after an extended 6-month course of daily proguanil therapy.33 The development and persistence of palpable splenomegaly in SCA warrants prospective evaluation to determine if intensified or prolonged malaria treatment might be helpful in reducing splenomegaly and lowering the incidence of subsequent malaria infections. We recognize that palpation is an imperfect measure of splenomegaly, but it is commonly used in Africa in clinical practice, based on the original Hackett grading scale.34 Future studies should include ultrasonography to measure spleen size and volume more accurately.
In contrast, several expected risk factors did not appear to be important for malaria within the REACH cohort. Although 1-4 cm splenomegaly was a risk factor, moderate to massive splenomegaly (≥5 cm) was not associated with higher incidence of malaria. Such large spleens in children usually develop from acute or chronic splenic sequestration crisis, which are characterized by large amounts of blood pooled within the red pulp parenchyma but do not necessarily reflect an expansion of macrophage populations that might phagocytose malaria. We hypothesize that multiple pathophysiological events can lead to splenomegaly in SCA within Africa, but these large spleens vary by size and function, which may explain the observed differential risks of malaria. Analyzing the incidence of malaria in the REACH cohort based on spleen size over time, and in the context of infections and splenic sequestration events, will be an important prospective research goal.
Somewhat surprisingly, HbF was not significantly associated with malaria risk. Previous studies have suggested that HbF within erythrocytes resists parasite invasion and growth, which helps explain the low rates of malaria observed in newborns.35 However, these studies used in vitro techniques36 or animal models37 for parasite invasion and were not prospective clinical studies conducted in humans. Further, a recent paper contradicts the notion that HbF within human erythrocytes is protective against parasite growth,38 which supports our observations in the REACH cohort. However, because those experiments were performed using erythrocytes with pancellular HbF distribution, they may not be predictive of the more heterocellular HbF distribution found in sickle erythrocytes.
Another potential mechanism of protection against malaria by hydroxyurea treatment is direct cytotoxicity to malaria parasites through its potent inhibition of ribonucleotide reductase and lowering of intracellular deoxynucleotide pools.39,40 Previous in vitro studies have documented inhibition of P falciparum growth after hydroxyurea exposure.41,42 Daily hydroxyurea in children with SCA may therefore inhibit parasite growth directly, with antimalarial properties analogous to its effects on HIV replication.43 Hydroxyurea’s effects on iron availability represent another intracellular mechanism by which treatment could protect against malaria infection. Malaria parasites require access to iron for their own Fe-sulfur–based enzymes and nucleic acid synthesis, and body iron stores have been shown to affect the rate of malaria infection and severity.44 Hydroxyurea is a potent inhibitor of ribonucleotide reductase in part through its effects as an iron chelator, disrupting the enzymatic activity, and thus can be considered to have direct antimalarial activity.45 Analyzing malaria risk in the REACH cohort based on the iron status of the individual study participants will be another important avenue of future investigation.
In summary, hydroxyurea at MTD for children with SCA is associated with a significantly lower incidence of malaria infections; this association is greater at higher hydroxyurea doses and is sustained over time. Potential mechanisms of action between hydroxyurea and malaria remain incompletely defined, but treatment effects in children with SCA related to myelosuppression, reduced inflammation, iron chelation, and antimalarial activity, in addition to general health improvement through higher Hb and HbF, could be important. Treatment-associated effects at MTD that target mild myelosuppression are especially salutary, and they justify using hydroxyurea at MTD to achieve mild myelosuppression with ANC <3.0 × 109/L.
Within Africa, awareness of SCA is increasing, along with an increased emphasis on the importance of improving health care for affected individuals. Many countries are moving from pilot newborn screening studies toward national testing programs, recognizing the need to identify all affected babies with SCA early in life.46 The American Society of Hematology has launched the Consortium on Newborn Screening in Africa with bold goals to improve sickle cell screening in many countries across sub-Saharan Africa.47 With improved diagnosis and preventive care, mortality rates for SCA within Africa are dropping rapidly,48,49 and wider access to hydroxyurea is the next logical step. Because malaria is documented to cause frequent and severe anemia in children with SCA,50 understanding the clinically significant association between hydroxyurea and lower malaria incidence represents an important research goal while expanding hydroxyurea treatment across Africa.
Acknowledgments
The authors thank the superb clinical teams from each of the REACH clinical sites in Luanda, Kinshasa, Kilifi, and Mbale, as well as early contributions of Patrick McGann to the trial. The authors also thank the patients and families who faithfully take hydroxyurea treatment every day and are committed to the REACH trial. They acknowledge the generous donation of hydroxyurea by Bristol Myers Squibb (BMS), provided through an investigator-sponsored research agreement (R.E.W.); BMS did not have any role in data review or data analysis and did not review the manuscript.
REACH is supported by U01 HL133883 from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (R.E.W.) and the Cincinnati Children’s Research Foundation. L.R.S. is supported by training grant K23 HL153763 from the NIH, National Heart, Lung, and Blood Institute. T.N.W. is funded through Senior Research Fellowships (202800 and 091758) from the Wellcome Trust.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article is published with the permission of the Director of Kenya Medical Research Institute.
Authorship
Contribution: P.O.-O., T.N.W., L.T., and B.S. collected the data and edited the manuscript; L.R.S. and B.A. helped design the analysis, analyzed the data, and edited the manuscript; K.M. and T.A.H. performed laboratory testing; S.E.S. and T.S.L. helped organize the study and edited the manuscript; G.T. and A.L. designed the analysis plan, performed the statistical analyses, and edited the manuscript; R.E.W. designed the study and analysis, analyzed the data, and wrote the first draft of the manuscript.
A complete list of the members of the REACH Investigators appears in “Appendix.”
Conflict-of-interest disclosure: R.E.W. receives research donations from Bristol Myers Squibb, Addmedica, and Hemex Health. The remaining authors declare no competing financial interests.
Correspondence: Russell E. Ware, Division of Hematology, 3333 Burnet Ave, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229; e-mail: russell.ware@cchmc.org.
References
Author notes
Data will be shared for requests from investigators whose proposed use has been approved formally by a review committee, and will be evaluated by the REACH Medical Coordinating Center and Data Management Center. After de-identification and with a signed data access agreement, individual participant data that underlie the results can be available for data meta-analysis, up to 36 months after publication.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal