TO THE EDITOR:
Patients with secondary acute myeloid leukemia (s-AML), a category which includes AML with myelodysplasia-related changes (AML-MRC) and treatment-related AML (t-AML), have poor long-term outcomes following standard induction chemotherapy (“7+3”).1,2 A previous population-based study demonstrated median survival of 6 to 7 months for patients with s-AML and 8 to 14 months for those with t-AML.1 In 2017, a liposomal cytarabine and daunorubicin formulation (CPX-351) was Food and Drug Administration (FDA) approved for upfront treatment of s-AML based on a pivotal phase 3 trial demonstrating improved overall survival (OS) (9.56 vs 5.95 months; hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.52-0.9) and remission rates (complete remission [CR]/CR with incomplete count recovery [CRi]) (47.7% vs 33.3%; P = .016) in patients aged 60 to 75 years old than induction chemotherapy with “7+3.”3 The benefits from therapy with CPX-351 were retained at the 5-year time point as well with median OS of 9.33 vs 5.95 months with “7+3.”4 CPX-351 treatment was subsequently approved for s-AML regardless of age. Here, we present safety and efficacy data in patients younger than 60 years old who were not eligible to be treated on this study. We sought to address the paucity of such data by retrospective review of clinical experience since FDA approval at 6 large academic centers.
Medical records were reviewed at each institute to identify all patients aged 18 to 59 years old with untreated s-AML defined as AML evolving from antecedent myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML), AML arising from previous cytotoxic or radiation therapy, or AML with World Health Organization–defined myelodysplasia-related changes (AML-MRC) treated with CPX-351 as induction therapy from August 2017 to December 2021. Variables including demographics, disease-specific variables, and outcomes were collected in accordance with the Roswell Park Institutional Review Board approved protocol and the Declaration of Helsinki. Responses to therapy were defined per 2003 International Working Group criteria,5 and comparison between groups was made using Fisher exact test (SPSS 28.0.1). Kaplan-Meier method was used to estimate the distribution of events over time. Time-to-events were evaluated using a stratified log-rank test to compare treatment groups. HRs and 95% CIs were estimated using a Cox proportional hazard model. GraphPad Prism, version 9.2.0 (GraphPad, La Jolla, CA) was used for statistical analysis.
A total of 66 patients with confirmed s-AML or t-AML treated with CPX-351 were included in this study. Median age was 54.9 years (range, 23-59), and 37 (56%) were male. The majority (N = 52, 79%) of patients had AML-MRC, and 14 (21%) had t-AML. Of the 66 patients, 16 had received previous hypomethylating therapy (HMA) for antecedent MDS. Cytogenetics were complex in 30 (46%), monosomal in 17 (26%), normal in 10 (15%), −7 in 7 (11%), +8 in 4 (6%), −17p in 3 (5%), and −5q in 2 (3%) patients. The most common mutations were TP53 (29%), RUNX1 (21%), DNMT3A (17%), NRAS (17%), ASXL1 (11%), and NPM1 (11%) (Table 1; supplemental Table 1, available on the Blood website).
Patient characteristics and outcomes
| Characteristics . | N (%) . |
|---|---|
| Total patients | 66 (100) |
| Age, median (range) (y) | 54.9 (23-59) |
| Gender | |
| Male | 37 (56) |
| Female | 29 (44) |
| AML subtype | |
| AML-MRC | 52 (79) |
| Morphology | 17 (26) |
| Cytogenetics | 11 (17) |
| Prior Dx MDS | 23 (35) |
| Prior Dx CMML | 1 (2) |
| Therapy-related AML | 14 (21) |
| Previous HMA treatment for MDS | |
| Yes | 16 (24) |
| No | 50 (76) |
| Baseline mutations of interest | |
| TP53 | 19 (30) |
| RUNX1 | 14 (22) |
| DNMT3A | 11 (17) |
| NRAS | 11 (17) |
| ASXL1 | 7 (11) |
| NPM1 | 7 (11) |
| FLT3 ITD | 5 (8) |
| IDH1 | 5 (8) |
| IDH2 | 3 (5) |
| Cytogenetics | |
| Complex | 30 (46) |
| Monosomal | 17 (26) |
| Normal | 10 (15) |
| Del 7 | 7 (11) |
| Trisomy 8 | 4 (16) |
| 17p | 3 (5) |
| Minus 5q | 2 (3) |
| Outcomes (out of 62 pts) | |
| CR | 19 (30.6) |
| CRi | 8 (12.9) |
| CR/CRi | 27 (43.5) |
| MLFS | 2 (3.2) |
| NR | 33 (53) |
| Mortality | |
| 30 d | 6 (9.1) |
| 60 d | 11 (16.7) |
| Characteristics . | N (%) . |
|---|---|
| Total patients | 66 (100) |
| Age, median (range) (y) | 54.9 (23-59) |
| Gender | |
| Male | 37 (56) |
| Female | 29 (44) |
| AML subtype | |
| AML-MRC | 52 (79) |
| Morphology | 17 (26) |
| Cytogenetics | 11 (17) |
| Prior Dx MDS | 23 (35) |
| Prior Dx CMML | 1 (2) |
| Therapy-related AML | 14 (21) |
| Previous HMA treatment for MDS | |
| Yes | 16 (24) |
| No | 50 (76) |
| Baseline mutations of interest | |
| TP53 | 19 (30) |
| RUNX1 | 14 (22) |
| DNMT3A | 11 (17) |
| NRAS | 11 (17) |
| ASXL1 | 7 (11) |
| NPM1 | 7 (11) |
| FLT3 ITD | 5 (8) |
| IDH1 | 5 (8) |
| IDH2 | 3 (5) |
| Cytogenetics | |
| Complex | 30 (46) |
| Monosomal | 17 (26) |
| Normal | 10 (15) |
| Del 7 | 7 (11) |
| Trisomy 8 | 4 (16) |
| 17p | 3 (5) |
| Minus 5q | 2 (3) |
| Outcomes (out of 62 pts) | |
| CR | 19 (30.6) |
| CRi | 8 (12.9) |
| CR/CRi | 27 (43.5) |
| MLFS | 2 (3.2) |
| NR | 33 (53) |
| Mortality | |
| 30 d | 6 (9.1) |
| 60 d | 11 (16.7) |
Dx, diagnosis; pts, patients; NR, nonresponder.
Most patients (N = 59, 89%) received one cycle of CPX-351 induction; 7 received 2 cycles (11%) (supplemental Table 2). At the time of analysis, response assessment was available for 62 patients. The overall complete response (CR/CRi) rate was 43.5% including 19 CR (30.6%) and 8 CR with incomplete count recovery (CRi, 12.9%). Two patients (3.2%) obtained morphologic leukemia-free state (MLFS) and the remainder (N = 33, 53%) did not respond (Table 1). Median duration of response from CR/CRi was 5.3 months (range, 0.5-14.2 months). A total of 31 patients (31/66, 47%) proceeded onto hematopoietic stem cell transplant (HSCT) after a median of 2 cycles within a median of 2.8 months. The CR/CRi rate among evaluable patients with TP53 mutated (TP53mut) AML was 31.6% (6/19), compared with 48.8% (21/41) in patients with TP53 wild type (TP53wt) (P = .26). However, median duration of remission was similar for patients with TP53wt and TP53mut (5.2 vs 5.4 months; P = .84). Patients with previous HMA exposure had a CR/CRi of 25% (4/16) vs patients who were HMA naïve (CR/CRi, 50%; P = .15) (supplemental Table 3).
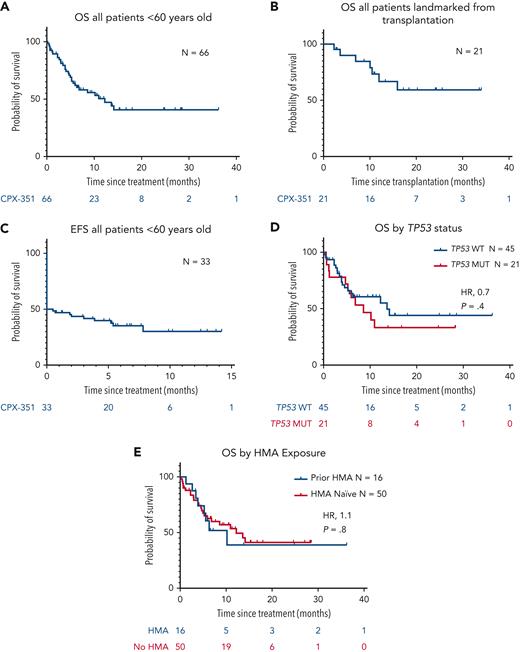
With a median follow-up of 12.4 months, the median OS in all 66 patients was 12.2 months (range, 0.2-36.2 months) (Figure 1A). In addition, landmark analysis of OS in 31 patients who underwent HSCT calculated from the time of transplantation demonstrated a median OS that was not reached (range, 2.3-34.1 months) (Figure 1B). Event-free survival (EFS) for all patients was not reached (range, 0.5-14.2 months) (Figure 1C). When stratifying for TP53mut, there were no differences in OS (median, 13.6 vs 8.6 months; P = .4) based on TP53 status (Figure 1D). In addition, we observed similar OS in patients who were HMA naïve and HMA exposed (10.2 vs 12.2 months; P = .8) (Figure 1E). Median time to count recovery was 37.2 and 39.8 days for neutrophils and platelets, respectively (supplemental Table 4). The most common adverse events included neutropenic fever (29/43, 67.4%) and 4 reports (9.3%) of clinically significant bleeding (supplemental Table 5). Early mortality was 9.1% at 30 days, and 16.7% at 60 days.
Kaplan-Meier estimates of median OS and EFS. (A) Median OS for all patients was found to be 12.2 months (range, 0.2-36.2 months). (B) Median OS landmarked from the date of transplantation was NR (range, 2.3-34.1 months). (C) Median EFS for all patients was NR (range, 0.5-14.2 months). (D) Median OS of patients with TP53wt vs TP53mut (13.6 vs 8.6 months). (E) Median OS of patients naïve to hypomethylating therapy (HMA) vs patients exposed to HMA (10.2 vs 12.2 months).
Kaplan-Meier estimates of median OS and EFS. (A) Median OS for all patients was found to be 12.2 months (range, 0.2-36.2 months). (B) Median OS landmarked from the date of transplantation was NR (range, 2.3-34.1 months). (C) Median EFS for all patients was NR (range, 0.5-14.2 months). (D) Median OS of patients with TP53wt vs TP53mut (13.6 vs 8.6 months). (E) Median OS of patients naïve to hypomethylating therapy (HMA) vs patients exposed to HMA (10.2 vs 12.2 months).
Overall, this multi-institutional retrospective analysis demonstrates comparable response rates (CR/CRi, 43.5%) and OS (12.2 months) for CPX-351 in younger patients (<60 years old) than older individuals in the phase 3 study (CR/CRi, 47.7%; median OS, 9.56 months) (Table 1).3 The majority (79%) of younger patients had a diagnosis of AML-MRC. These outcomes suggest the underlying biology of s-AML impacts outcome more than age. Despite lower CR/CRi rates in TP53 mutant AML and in patients with previous HMA, OS for patients were similar regardless of TP53 status or previous therapy. Historically, patients <65 years old with s-AML had a reported OS of approximately 7 months. Our data demonstrate that CPX-351 followed by HSCT may improve outcomes of younger patients with s-AML as compared with previous studies.
The authors acknowledge that as a retrospective study other unaccounted factors may contribute to our observed OS. To date, HSCT has been a mainstay of therapy for patients with s-AML, offering the only curative option.6-10 This is supported by our landmark OS analysis where median OS was not reached among those who went to transplant. Consistent with our observation, Matthews and colleagues evaluated real-world outcomes for patients with AML induced with CPX-351 or azacitidine/venetoclax.11 They demonstrated superior survival in those who underwent HSCT irrespective of induction strategy. This work highlights the crucial role of consolidation with HSCT in this patient population following the achievement of therapeutic response to chemotherapy. However, lack of response following CPX-351 in patients with previous HMA exposure (69%) or TP53 mutation (63%) preclude HSCT; for these individuals, clinical trials of agents such as venetoclax, eprenetapopt, magrolimab, or other immunotherapy remain a high priority.
The authors acknowledge that this study is limited by the small number of patients, retrospective study design, and short duration of follow-up. A phase II study prospectively evaluating CPX-351 in patients <60 years old with s-AML is ongoing and open to accrual (NCT04269213). Although such patients are traditionally considered better risk than their older counterparts considering their “youth” and fitness for intensive chemotherapy, our study highlights the poor survival outcome in younger patients with s-AML who are unable to proceed to HSCT. Although our data show somewhat improved outcomes for young patients with s-AML bridged to HSCT with CPX-351, overall outcomes in this high-risk population remain sobering and emphasize the need to accelerate development of novel therapeutic approaches.
Patients provided written informed consent for therapy with CPX-351, and the research was conducted in accordance with the Roswell Park Comprehensive Cancer Center Institutional Review Board approved protocol and the Declaration of Helsinki.
Acknowledgments
The authors gratefully acknowledge the support of the Roswell Park Alliance Foundation (Jacquie Hirsch Leukemia Research Fund to E.S.W.). Core resource support was provided by the National Institutes of Health, National Cancer Institute Cancer Center Support Grant for Roswell Park Cancer Institute (CA016156).
Authorship
Contribution: A.P. and E.S.W. designed and conceived the study; A.P., A.D.G., C.T., S.F., P.V., S.S., J.W., B.B., C.F., and M.S. identified patients and performed clinical annotation; A.P., A.D.G., S.F., P.V., S.T., J.B., E.A.G., J.E.T., K.S., and E.S.W. analyzed and interpreted the data; and A.P. and E.S.W. wrote the manuscript with assistance from all other authors.
Conflict-of-interest disclosure: A.P. receives research funding from Jazz Pharmaceuticals. A.D.G. receives research funding from Pfizer, Prelude Therapeutic, Celularity; provides consultancy and receives research funding from Aptose; provides consultancy, is a member on an entity’s board of directors or advisory committees, and receives research funding from AbbVie; receives research funding from Aprea and AROG; provides consultancy and is a member on an entity’s board of directors or advisory committees for Genentech and Astellas Pharma; and receives honoraria from DAVA Oncology. C.T. is on the speakers bureau for Astellas Pharma, and Jazz Pharmaceuticals, and receives honoraria from Pfizer, BMS, and AbbVie. S.F. provides consultancy, receives honoraria from, and is on the speakers bureau for Takeda, Stemline Therapeutics, Taiho Pharmaceuticals, Sanofi Genzyme, Novartis, Karyopharm Pharmaceuticals, Jazz Pharmaceuticals, Janssen Oncology, Incyte, GlaxoSmithKline, Gilead Sciences, Bristol Myers Squibb, Amgen, and Agios. P.V. provides consultancy and is on the speakers bureau for Incyte; provides consultancy for Pfizer, Blueprint Medicines, Jazz Pharmaceuticals, Novartis, AbbVie, CTI BioPharma Corp, and Agios; is a current employee at O'Neal Comprehensive Cancer Center, The University of Alabama at Birmingham; receives research funding from Seattle Genetics; and is on the speakers bureau for Astellas Pharma. E.A.F. receives honoraria from Physician Educational Resource, MediCom Worldwide, American Society of Hematology, Picnic Health, AAMDSIF, and Dresner Foundation; provides consultancy and is on the advisory board for CTI BioPharma; receives research funding from Blueprint Medicines and Celldex Therapeutics; receives research funding from, provides consultancy, and is on the advisory board for Genentech; provides consultancy and is on the advisory board for AstraZeneca Rare Disease; provides consultancy for Boston Biomedical; provides consultancy and receives honoraria from Taiho Oncology; receives honoraria and research funding from Novartis; provides consultancy for and receives honoraria from Takeda Oncology; receives honoraria and research funding from Astex Pharmaceuticals; provides consultancy for and receives honoraria from AbbVie; provides consultancy, is on the advisory board for, and receives honoraria and research funding from Celgene/Bristol Myers Squibb; receives research funding, provides consultancy for, and is on the advisory board for Apellis Pharmaceuticals; and provides consultancy for and receives research funding from Alexion Pharmaceuticals. J.E.T. receives research funding from Novartis/Bristol Myers Squibb. K.S. is a member on an entity’s board of directors or advisory committees for Gilead and AROG; receives honoraria and is a member on an entity's board of directors or advisory committees for Novartis; provides consultancy and is a member on an entity’s board of directors or advisory committees for Astellas Pharma; and receives honoraria and is a member on an entity’s board of directors or advisory committees for Bristol Meyers Squibb. E.S.W. provides consultancy, receives honoraria, and is a member on the advisory board and speakers bureau for Stemline Therapeutics and Pfizer; provides consultancy and receives honoraria from Mana Therapeutics; provides consultancy, receives honoraria, and is on the advisory board for Novartis; provides consultancy, receives honoraria, and is on the advisory board, steering committee, and speakers bureau for Kura Oncology; provides consultancy, receives honoraria, and is on the advisory board for Kite Pharmaceuticals; provides consultancy, receives honoraria, and is on the advisory board for Jazz Pharmaceuticals; provides consultancy, receives honoraria, and is on the advisory board for GlaxoSmithKline; is on the advisory committees for Genentech and BMS/Celgene; provides consultancy and is on the advisory committees for Astellas and AbbVie; provides consultancy, receives honoraria, and is on the advisory board for Takeda; provides consultancy and is on the speakers bureau for DAVA Oncology; is on the data safety monitoring committee for Rafael Pharmaceuticals; provides consultancy, receives honoraria, and is on the advisory board for Gilead; provides consultancy, receives honoraria, and is on the advisory board for Daiichi Sankyo and PTC Therapeutics; and provides consultancy for Genentech and MacroGenics. The remaining authors declare no competing financial interests.
Correspondence: Amanda Przespolewski, Department of Medicine, Leukemia Service, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263; e-mail: amanda.przespolewski@roswellpark.org.
References
Author notes
Data are available on request from the corresponding author, Amanda Przespolewski (amanda.przespolewski@roswellpark.org).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal