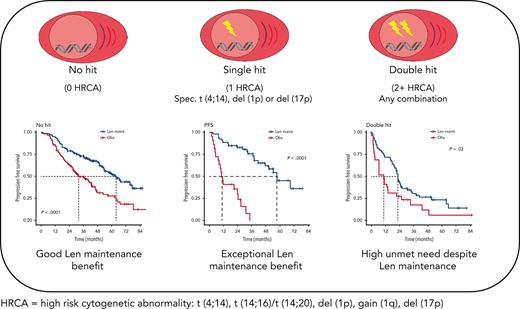
Key Points
Patients with myeloma with isolated del(1p), del(17p), or t(4;14) derive exceptional benefit from Len maintenance after transplantation.
Patients with double-hit myeloma are in urgent need of novel maintenance approaches, regardless of the high-risk aberrations involved.
Abstract
Prediction of individual patient benefit from lenalidomide (Len) maintenance after autologous stem cell transplant (ASCT) remains challenging. Here, we investigated extended molecular profiling for outcome prediction in patients in the National Cancer Research Institute Myeloma XI (MyXI) trial. Patients in the MyXI trial randomized to Len maintenance or observation after ASCT were genetically profiled for t(4;14), t(14;16), t(14;20), del(1p), gain(1q), and del(17p) and co-occurrence of risk markers was computed. Progression-free survival (PFS), subsequent progression (PFS2), and overall survival (OS) were calculated from maintenance randomization, and groups were compared using Cox proportional hazards regression. Of 556 patients, 17% with double-hit multiple myeloma (MM) (≥2 risk markers), 32% with single-hit (1 risk marker), and 51% without risk markers were analyzed. Single-hit MM derived the highest PFS benefit from Len maintenance, specifically, isolated del(1p), del(17p), and t(4;14), with ∼40-fold, 10-fold, and sevenfold reduced risk of progression or death (PFS), respectively, compared with observation. This benefit translated into improved PFS2 and OS for this group of patients compared with observation; median PFS was 10.9 vs 57.3 months for observation vs Len maintenance. Patients with isolated gain(1q) derived no benefit, and double-hit MM limited benefit (regardless or risk lesions involved) from Len maintenance. Extended genetic profiling identifies patients deriving exceptional benefit from Len maintenance and should be considered for newly diagnosed patients to support management discussions along their treatment pathway. This trial was registered at www.isrctn.com/ISRCTN49407852 as # ISRCTN49407852.
Introduction
For patients with newly diagnosed multiple myeloma (NDMM) lenalidomide (Len) is the standard therapy and, presently, the only licensed post–autologous stem cell transplantation (ASCT) maintenance therapy.1-3 Side effects associated with Len maintenance include cytopenia, vascular events, diarrhea/bile acid malabsorption, and an increased risk of second malignancy.1,4-6 Although side effects from long-term treatment with Len are manageable in most patients, continuous therapy can be a physical and psychological challenge for some, raising questions about its benefit for individual patients.7 In addition, financial toxicity of ongoing Len maintenance can lead to queries about treatment breaks or discontinuation.8,9
Len maintenance has consistently been associated with improved progression-free survival (PFS) in randomized phase 3 trials and overall survival (OS) in the UK National Cancer Research Institute Myeloma XI (MyXI) trial as well as in a recent meta-analysis.3,6 However, the interpatient heterogeneity in PFS and OS in trials and in clinical practice is consistently large and estimating the probability of an individual patient benefiting from Len maintenance is not straightforward.
To better identify which patients are likely to gain maximal benefit from Len maintenance, we investigated outcomes of patients in the MyXI trial from maintenance randomization: Len maintenance vs observation, in the context of extended tumor genetic profiles, including co-occurrence of high-risk genetic lesions.
Material and methods
Patients in the MyXI trial
Patients with NDMM (n = 556) enrolled in the MyXI phase 3 trial and post-ASCT randomized to maintenance or observation underwent extended genetic profiling. This included profiling of copy number aberrations: del(1p), del(17p), and gain(1q); adverse translocations: t(4;14), t(14;16), and t(14;20); and their co-occurrence, as in, no hit (no adverse lesion; standard risk), single hit (1 adverse lesion; high risk), and double hit (≥2 adverse lesions; ultrahigh risk). For all patients, myeloma tumor cells were immunomagnetically purified (>95%) from baseline bone marrow using anti-CD138 antibodies (Miltenyi Biotec, Bergisch Gladbach, Germany) and copy number aberrations and immunoglobulin translocations profiled using multiplexed ligation–dependent probe amplification (P425-B1 MM probe mix; MRC Holland, Amsterdam, The Netherlands) and quantitative reverse transcription polymerase chain reaction, which have been extensively validated against fluorescence in situ hybridization, as previously described.10,11
The design and main outcomes of the MyXI trial have been reported previously.6 Briefly, patients who were transplant eligible were randomized to induction therapy with cyclophosphamide, dexamethasone combined with thalidomide; cyclophosphamide, Len, and dexamethasone; or carfilzomib combined with cyclophosphamide, Len, and dexamethasone; and underwent ASCT. Hundred days post-ASCT, eligible patients underwent further randomization to Len, Len/vorinostat, or observation. In this current analysis, patients randomized to Len or Len/vorinostat were analyzed jointly. The study was undertaken with written informed consent from patients, and ethical approval was obtained from the Oxfordshire Research Ethics Committee (MREC 17/09/09, #ISRCTN49407852).
Statistical methods
All statistical analyses were undertaken using R version 4.0.5 and the survival, survminer, and gtsummary packages. PFS was defined as the time from the date of maintenance randomization to PFS or subsequent progression (PFS2), according to International Myeloma Working Group criteria, or death from any cause. OS was defined as the time from the date of maintenance randomization to death from any cause. Cox regression analysis was used to estimate hazard ratios (HRs) and respective 95% confidence intervals (CIs).
Results
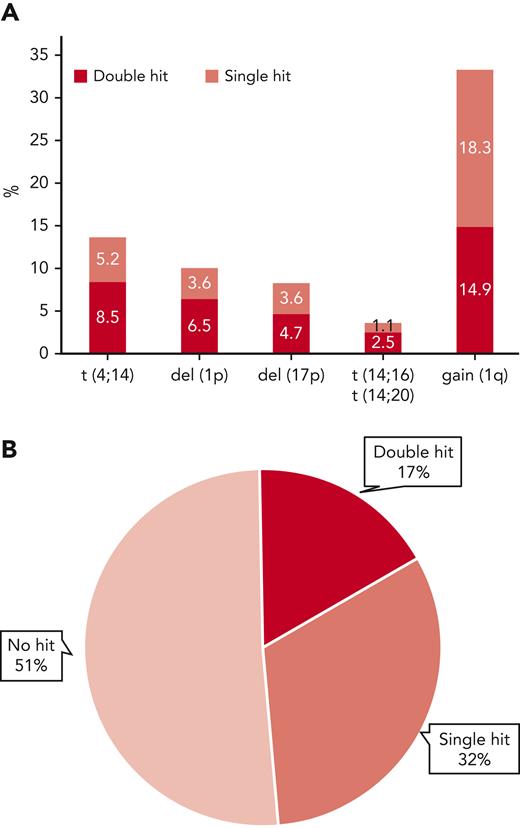
Extended genetic profiles were available for 556 patients in the MyXI trial randomized to Len maintenance or observation, with a median follow-up of 51.6 months (interquartile range, 37.3-68.0 months) from maintenance randomization. Inclusion in the current study was solely based on availability of central genetic baseline bone marrow material for extended profiling. The study population is reflective of the overall patient population that was eligible for transplantation in the MyeXI trial (n = 1444). Included patients were recruited from 94 of a total of 105 participating UK National Health Service hospitals that provided nonmandatory patient samples for translational studies in MyXI. Clinical characteristics and frequencies of individual genetic lesions were comparable with the previously published overall transplant-eligible group (Table 1). Treatment randomization from inclusion in the study (supplemental Table 1, available on the Blood website), as well as PFS and OS for the 556 patients in context of maintenance randomization was also reflective of the entire patient population that was eligible for transplantation.6 Co-occurrence of ≥2 lesions was frequent, as expected, 62% of t(4;14) tumors (47/76) carried at least a second hit, as did 64% of del(1p) (35/36), 57% of del(17p) (26/46), and 45% of gain(1q) (83/185) tumors (Figure 1). Double hit was most frequent in t(14;16)/t(14;20) tumors at 70%; however, t(14;16)/t(14;20) was infrequent, as expected (n = 20; 3.6%). Accordingly, there were very few patients with single hit, which did not allow for separate statistical analysis. Overall, 32% of tumors showed single hit, 17% double hit, and 51% showed no high-risk aberration (Figure 1). Premaintenance response rates were comparable between these 3 molecular risk groups (supplemental Table 2).
Baseline clinical and genetic characteristics of patients in the MyXI trial
| Characteristic . | N . | N = 556∗ . |
|---|---|---|
| Age, y | 556 | 59 (54-64) |
| Male | 556 | 360 (65) |
| World Health Organization performance status | 534 | |
| 0 | 233 (44) | |
| 1 | 214 (40) | |
| 2 | 65 (12) | |
| ≥3 | 22 (4.1) | |
| Unknown | 22 | |
| International Staging System | 519 | |
| 1 | 167 (32) | |
| 2 | 240 (46) | |
| 3 | 112 (22) | |
| Unknown | 37 | |
| Revised International Staging System | 445 | |
| 1 | 78 (17) | |
| 2 | 314 (71) | |
| 3 | 53 (12) | |
| Unknown† | 111 | |
| Kappa light chain restriction | 553 | 363 (66) |
| Unknown | 3 | |
| Induction randomization | 556 | |
| KCRD | 122 (22) | |
| CTD | 210 (38) | |
| CRD | 224 (40) | |
| Maintenance randomization | 556 | |
| Len | 359 (65) | |
| Observation | 197 (35) | |
| t(4;14) | 556 | 76 (14) |
| t(14;16)/t(14;20) | 556 | 20 (3.6) |
| del(1p) | 556 | 56 (10) |
| del(17p) | 556 | 46 (8.3) |
| gain(1q) | 556 | 185 (33) |
| Characteristic . | N . | N = 556∗ . |
|---|---|---|
| Age, y | 556 | 59 (54-64) |
| Male | 556 | 360 (65) |
| World Health Organization performance status | 534 | |
| 0 | 233 (44) | |
| 1 | 214 (40) | |
| 2 | 65 (12) | |
| ≥3 | 22 (4.1) | |
| Unknown | 22 | |
| International Staging System | 519 | |
| 1 | 167 (32) | |
| 2 | 240 (46) | |
| 3 | 112 (22) | |
| Unknown | 37 | |
| Revised International Staging System | 445 | |
| 1 | 78 (17) | |
| 2 | 314 (71) | |
| 3 | 53 (12) | |
| Unknown† | 111 | |
| Kappa light chain restriction | 553 | 363 (66) |
| Unknown | 3 | |
| Induction randomization | 556 | |
| KCRD | 122 (22) | |
| CTD | 210 (38) | |
| CRD | 224 (40) | |
| Maintenance randomization | 556 | |
| Len | 359 (65) | |
| Observation | 197 (35) | |
| t(4;14) | 556 | 76 (14) |
| t(14;16)/t(14;20) | 556 | 20 (3.6) |
| del(1p) | 556 | 56 (10) |
| del(17p) | 556 | 46 (8.3) |
| gain(1q) | 556 | 185 (33) |
CTD, cyclophosphamide, dexamethasone combined with thalidomide; CRD, cyclophosphamide, Len, and dexamethasone; KCRD, carfilzomib combined with CRD.
Statistics presented: median (interquartile range) or n (%).
Main reason for missingness is missing lactate dehydrogenase test.
Frequency of individual high-risk genetic lesions and their co-occurrence. (A) Frequency of high-risk genetic lesions, bar height represents overall frequency, divided by isolated occurrence (single hit) or co-occurrence with ≥1 of the other lesions (double hit) by color code. (B) Pie diagram showing overall frequencies of no, single, or double hit, the latter also including tumors with ≥3 hits.
Frequency of individual high-risk genetic lesions and their co-occurrence. (A) Frequency of high-risk genetic lesions, bar height represents overall frequency, divided by isolated occurrence (single hit) or co-occurrence with ≥1 of the other lesions (double hit) by color code. (B) Pie diagram showing overall frequencies of no, single, or double hit, the latter also including tumors with ≥3 hits.
Len maintenance was associated with longer PFS (HR, 0.50; 95% CI, 0.40-0.62; P < .0001) and longer PFS2 (HR, 0.65; 95% CI, 0.50-0.86; P = .002) compared with observation in this group of patients in the MyXI trial (supplemental Figure 1). OS was nominally longer for Len maintenance, however, not significantly different (HR, 0.8; 95% CI, 0.59-1.10; P = .17), very likely because of the smaller number of patients included in this subgroup analysis as compared with the previously published overall transplant-eligible population in which the significance threshold was met.6 Median PFS was 53.8 months (95% CI, 49.0-63.8) for Len maintenance vs 27.3 months (95% CI, 22.8-34.3) for observation, and median OS for Len maintenance was 80.7 months (95% CI, 76.4 to not evaluable [NE]) vs 78.5 months (95% CI, 70.3-NE), consistent with previously published outcomes for MyXI maintenance randomization overall.6
Stratifying patients by genetic profile, Len maintenance had the biggest effect on PFS in patients with single hit (HR, 0.38; 95% CI, 0.25-0.58; P < .0001), followed by patients with no hit (HR, 0.46; 95% CI, 0.33-0.64; P < .0001) and those with double hit (HR, 0.55; 95% CI, 0.34-0.90; P = .017) (supplemental Figure 2).
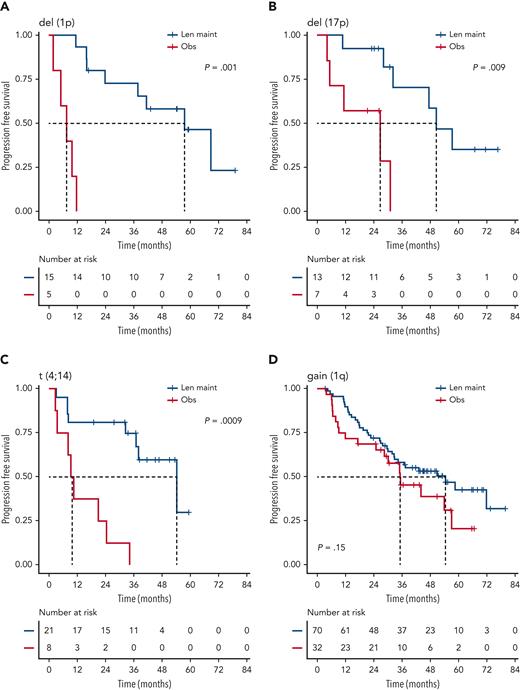
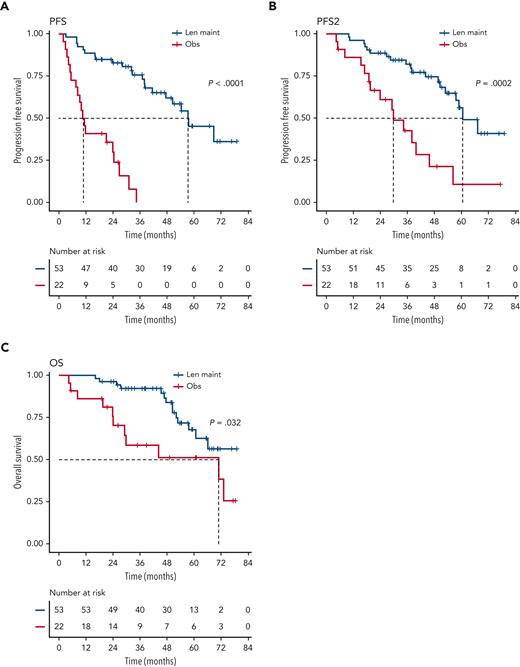
We investigated the single-hit group in more detail, finding that patients with isolated del(1p), del(17p), and t(4;14), in particular, benefited from Len maintenance, with HRs for PFS of 0.02 (95% CI, 0.002-0.24; P = .0012), 0.1 (95% CI, 0.02-0.58; P = .0095), and 0.14 (95% CI, 0.04-0.45; P = .0009), respectively (Figure 2). Patients with isolated del(1p) or t(4;14) randomized to observation experienced very early relapses after ASCT, with median PFS from randomization of 7.5 months (95% CI, 5.0-NE) and 9.9 months (95% CI, 8.2-NE), respectively (Figure 2). In contrast, patients with del(1p) or t(4;14) randomized to Len maintenance had a median PFS, not dissimilar from the overall group, of 57.6 months (95% CI, 37.8-NE) and 54.3 months (95% CI, 36.9-NE), respectively. For del(17p), the median PFS for patients in the observation group was 26.7 months (95% CI, 5.32-NE) compared with 50.5 months (95% CI, 32.2-NE) in the Len maintenance group. As group sizes were small, we jointly analyzed del(1p), del(17p), and t(4;14) groups for PFS2 and OS. Len maintenance was associated with longer PFS2 with a median of 60.5 months (95% CI, 57.6-NE) vs 29.7 months (95% CI, 19.4-NE) and a HR of 0.27 (95% CI, 0.13-0.54; P = .0002) and longer median OS that was not reached (95% CI, 60.7-NE) vs 70.8 months (95% CI, 29.1-NE) for observation, with a HR of 0.41 (95% CI, 0.18-0.93; P = .032) (Figure 3). In comparison, median PFS2 for patients without high-risk lesions was not reached (95% CI, 52.7-NE) for Len maintenance vs 78.5 months (95% CI, 72.6-NE) for observation with a HR of 0.56 (95% CI, 0.36-0.88; P = .01) and median OS not reached (95% CI, NE for both) vs 82.4 months (95% CI, 78.5-NE) with a HR of 0.69 (95% CI, 0.41-1.16; P = .2), respectively (supplemental Figure 2).
PFS of patients with isolated high-risk lesions by maintenance randomization. Kaplan-Meier plots for PFS for patients with isolated/single-hit del(1p) (A), del(17p) (B), t(4;14) (C), or gain(1q) (D) for randomization groups for Len maintenance (maint) (blue) or observation (Obs) (red); P values: Wald-P.
PFS of patients with isolated high-risk lesions by maintenance randomization. Kaplan-Meier plots for PFS for patients with isolated/single-hit del(1p) (A), del(17p) (B), t(4;14) (C), or gain(1q) (D) for randomization groups for Len maintenance (maint) (blue) or observation (Obs) (red); P values: Wald-P.
Outcome of patients with single-hit del(1p), del(17p), or t(4;14). Kaplan-Meier plots for PFS (A), PFS2 (B), and OS (C) for patients randomized to Len maintenance (blue curves) or observation (red curves); P values: Wald-P.
Outcome of patients with single-hit del(1p), del(17p), or t(4;14). Kaplan-Meier plots for PFS (A), PFS2 (B), and OS (C) for patients randomized to Len maintenance (blue curves) or observation (red curves); P values: Wald-P.
Patients with single-hit gain(1q) did not experience a significant improvement in PFS (HR, 1.5; 95% CI, 0.9-2.7; P = .2) or OS (HR, 1.1; 95% CI, 0.5-2.4; P = .9) with Len maintenance compared with observation (Figure 2). However, patients with gain(1q) did not relapse as early after ASCT as patients with del(1p) or t(4;14) when randomized to observation, with a median PFS of 35.1 months (95% CI, 28.5-NE) vs 54.4 months (95% CI, 32.8-NE) for Len maintenance. When analyzing outcomes in relation to 1q copy number, there was no differential outcome in Len maintenance vs observation when comparing groups with either 3 or ≥4 (amp[1q]) copies of 1q (supplemental Figure 3).
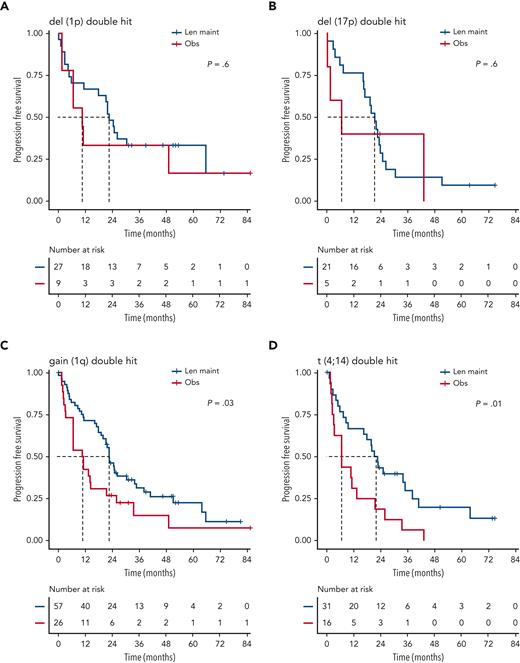
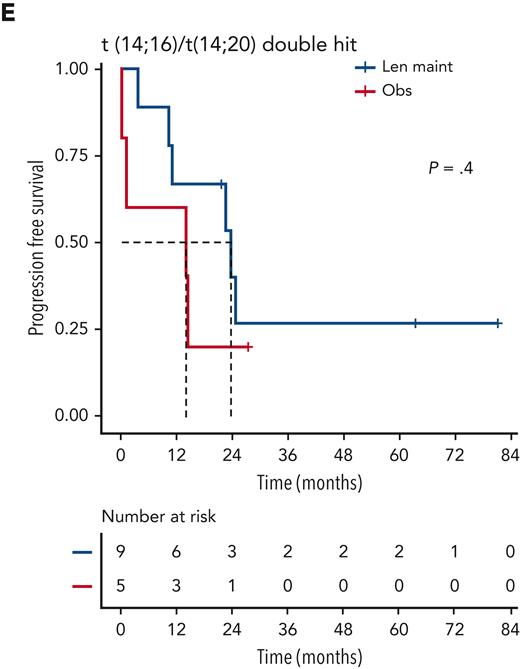
Although nominally benefiting from Len maintenance, outcome was generally poorest for patients with double-hit multiple myeloma (MM). Median PFS for Len maintenance was 22.5 months (95% CI, 20.0-30.4) vs 10.6 months (95% CI, 6.4-21.4) for observation, with a HR of 0.55 (95% CI, 0.34-0.90; P = .02) and median OS of 47.3 months (95% CI, 34.3-NE) vs 32.8 months (95% CI, 29.6-NE) and a HR of 0.88 (95% CI, 0.48-1.61; P = .7), respectively (supplemental Figure 2). To investigate whether outcome might differ for some patients with double hit, we considered genetic subgroups in which all tumors in the respective subgroup carried either gain(1q), del(1p), del(17p), t(4;14), or t(14;16)/t(14;20). Median PFS was consistently <24 months for all subgroups of patients with double hit, even those randomized to Len maintenance (Figure 4).
Outcome of subgroups of patients with double-hit disease, defined by unifying lesions. Kaplan-Meier plots for PFS for double-hit subgroups defined by presence of del(1p) (A), del(17p) (B), gain(1q) (C), t(4;14) (D), and t(14;16)/t(14;20) (E); P values: Wald-P.
Outcome of subgroups of patients with double-hit disease, defined by unifying lesions. Kaplan-Meier plots for PFS for double-hit subgroups defined by presence of del(1p) (A), del(17p) (B), gain(1q) (C), t(4;14) (D), and t(14;16)/t(14;20) (E); P values: Wald-P.
Discussion
Our findings show, to our knowledge for the first time, a molecularly characterized subgroup of patients who derive exceptional PFS and OS benefit from Len maintenance. Extended assessment of genetic markers enables the separation of these patients from those with double-hit tumors, who, unfortunately, continue to have unsatisfactory outcomes. Although reporting of genetic subgroups has traditionally been limited to t(4;14) and del(17p), our data strongly support wider access to extended profiling for patients with NDMM. Such profiling should afford more individualized use of Len maintenance for patients, and help identify patients with unmet need, who have a requirement for closer monitoring and, potentially, use of novel approaches. Diagnostically developed molecular tests optimized for detection of structural aberrations, such as multiplexed ligation–dependent probe amplification used in this study, rather than over fluorescence in situ hybridization, can potentially help in generating extended genetic profiles in a clinical setting.11
Our results also suggest that there is limited benefit of Len maintenance for patients with isolated gain(1q). Gain(1q) is emerging as a key driver with ongoing tumor evolutionary relevance in MM, in contrast with del(1p) or del(17p), which involve loss of tumor suppressor genes CDKN2C and TP53, respectively, or t(4;14), which is an early event in MM pathogenesis.12,13 We recently demonstrated poor outcome for evolving gain(1q) at relapse, an evolutionary hotspot in MM.14 Current results support further investigation of gain(1q) in primary and secondary resistance to immunomodulatory drug therapy.
MyXI is the only phase 3 trial that compared Len maintenance against observation for which extended genetics were assessed in a larger number of patients.3 Most ongoing trials for patients with NDMM randomize patients to single (mostly Len maintenance) vs doublet maintenance therapy. Accordingly, patients with isolated del(1p), del(17p), or t(4;14) may show outcomes similar to patients at standard risk in many future trials for NDMM. The high risk of early relapse on observation shown here should be considered in such patients keen to explore treatment-free intervals. Results from the recently published GMMG-HD5 study suggest that an increased relapse risk is maintained in patients with risk lesions, even after achieving complete remission with Len maintenance.15 Whether this is a post-ASCT or high-dose melphalan-specific effect, as previously suggested for del(1p),16 or whether these patients also have an increased relapse risk with other therapies such as chimeric antigen receptor T-cell therapy or response/minimal residual disease–guided treatment cessation, warrants further investigation.
Furthermore, our results demonstrate that regardless of the type of genetic lesions involved and including del(1p),17 double hit is consistently associated with poor outcome. Patients with double hit are clearly in urgent need of novel effective treatment approaches, especially those that maintain longer term responses. Exploratory data from the FORTE trial, as well as prospective data from OPTIMUM/MUKnine have recently shown promising early signs for improved outcomes with intensified post-ASCT maintenance for such patients.18,19 These data specifically highlight that comprehensive profiling and correct identification of double hit at presentation is required to adapt later, post-ASCT treatment to risk.
Our results strongly support the use of continuous Len maintenance after ASCT in patients with NDMM with isolated risk markers del(1p), del(17p), or t(4;14), for whom this therapy is associated with marked prolongation of PFS and OS. To identify these patients, but also those with double hit, who are in need of novel maintenance approaches, extended profiling at diagnosis should be considered for all patients with NDMM who are eligible for transplantation, with a minimum requirement of t(4;14), t(14;16)/t(14;20), del(1p), gain(1q), and del(17p) assessment, all of which are clinically accessible.
Acknowledgments
The authors thank all participating investigators, centers, the participating patients, and their families. The authors are grateful for the UK Myeloma Research Alliance and the National Cancer Research Institute Haematological Oncology Clinical Studies Group.
This work was supported by research grants from Myeloma UK, a Jacquelin Forbes-Nixon Fellowship (M.F.K.), and infrastructure support from the National Institute of Health Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research, London. Primary financial support for National Cancer Research Institute Myeloma XI was provided by Cancer Research UK (C1298/A10410). In addition, this work was supported by Core Clinical Trials Unit Infrastructure from Cancer Research UK (C7852/A25447) (D.A.C.).
Authorship
Contribution: M.F.K., R.H., and A.P. designed the study and analyzed the data; M.F.K. and A.P. wrote the manuscript; and all authors collected and curated data and revised and approved the manuscript.
Conflict-of-interest disclosure: D.A.C. received research funding from Celgene Corporation, Amgen, Merck Sharp, and Dohme. C.P. received consulting fees, honoraria, and travel support from Amgen, Celgene Corporation, Janssen, Sanofi, and Takeda Oncology. G.C. received consulting fees and honoraria for Janssen, Bristol Myers Squibb (BMS), Amgen, Takeda, Karyopharm Therapeutics, and Oncopeptides; honoraria from Jazz Pharmaceuticals; and research support from Takeda and Celgene. K.B. received consultancy fees and honoraria from Janssen, Celgene/BMS, Sanofi, and Takeda Oncology. F.E.D. received honoraria and consulting fees from Celgene/BMS, Takeda, Sanofi, GSK, Janssen, Oncopeptides, Amgen, and AbbVie. M.J. received honoraria from Janssen Oncology, Takeda, and Celgene/BMS and consulted for Janssen Oncology, Takeda, AbbVie, Sanofi, and Celgene/BMS. G.J.M. received consultancy fees, honoraria, and travel support from Amgen, Celgene Corporation, Janssen, Sanofi, and Takeda Oncology. R.O. received honoraria from Janssen Oncology, BeiGene, and AstraZeneca and consulting fees from BeiGene and Janssen Oncology. G.J. received research funding from Takeda and Celgene/BMS and honoraria for speaking from Takeda, Celgene/BMS, Amgen, Janssen, Sanofi, and Oncopeptides. M.D. owns stock in Abbingdon Health. M.F.K. has consulted for AbbVie, GSK, Karyopharm, Pfizer, and Seattle Genetics; consulted for and received honoraria from Amgen; consulted for and received honoraria, research support, and travel support from Celgene/BMS; and consulted for and received honoraria and travel support from Janssen and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Martin F. Kaiser, Division of Genetics and Epidemiology, Institute of Cancer Research, 123 Old Brompton Rd, London, SW7 3RP, United Kingdom; e-mail: martin.kaiser@icr.ac.uk.
References
Author notes
Data are available on request from the corresponding author, Martin F. Kaiser (martin.kaiser@icr.ac.uk). Only methodologically sound proposals whose proposed use of the data has been approved by the independent trial steering committee will be considered. Following approval, data requestors will need to sign a data access agreement.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal