Key Points
A risk-stratified, response-adapted salvage strategy resulted in high CMR rates with limited toxicities in CAYA with R/R cHL.
CMR rate after nivo + BV induction was 59% and increased to 94% with BV + bendamustine intensification.
Abstract
Children, adolescents, and young adults (CAYA) with relapsed/refractory (R/R) classic Hodgkin lymphoma (cHL) without complete metabolic response (CMR) before autologous hematopoietic cell transplantation (auto-HCT) have poor survival outcomes. CheckMate 744, a phase 2 study for CAYA (aged 5-30 years) with R/R cHL, evaluated a risk-stratified, response-adapted approach with nivolumab plus brentuximab vedotin (BV) followed by BV plus bendamustine for patients with suboptimal response. Risk stratification was primarily based on time to relapse, prior treatment, and presence of B symptoms. We present the primary analysis of the standard-risk cohort. Data from the low-risk cohort are reported separately. Patients received 4 induction cycles with nivolumab plus BV; those without CMR (Deauville score >3, Lugano 2014) received BV plus bendamustine intensification. Patients with CMR after induction or intensification proceeded to consolidation (high-dose chemotherapy/auto-HCT per protocol). Primary end point was CMR any time before consolidation. Forty-four patients were treated. Median age was 16 years. At a minimum follow-up of 15.6 months, 43 patients received 4 induction cycles (1 discontinued), 11 of whom received intensification; 32 proceeded to consolidation. CMR rate was 59% after induction with nivolumab plus BV and 94% any time before consolidation (nivolumab plus BV ± BV plus bendamustine). One-year progression-free survival rate was 91%. During induction, 18% of patients experienced grade 3/4 treatment-related adverse events. This risk-stratified, response-adapted salvage strategy had high CMR rates with limited toxicities in CAYA with R/R cHL. Most patients did not require additional chemotherapy (bendamustine intensification). Additional follow-up is needed to confirm durability of disease control. This trial was registered at www.clinicaltrials.gov as #NCT02927769.
Introduction
Most children, adolescents, and young adults (CAYA) with classic Hodgkin lymphoma (cHL) can be cured with first-line treatment; however, CAYA with relapsed/refractory (R/R) cHL have a 10-year progression-free survival (PFS) rate of just 47%.1 Despite therapeutic advances and the development of multiple salvage regimens, the largest retrospective review of outcomes for CAYA patients with R/R Hodgkin lymphoma (HL) who received autologous hematopoietic cell transplantation (auto-HCT) reported relapse rates as high as 41% at 5 years1; therefore, novel therapeutic approaches are needed.
Response to salvage therapy before auto-HCT, as determined by fluorodeoxyglucose positron emission tomography (FDG-PET), is a strong predictor of outcomes after auto-HCT in CAYA with R/R cHL.2,3 Adult patients with FDG-PET–negative disease before auto-HCT have significantly better event-free survival than patients with FDG-PET–positive disease at the time of auto-HCT,4 and current treatment guidelines for younger patients with R/R cHL consider a lack of complete metabolic response (CMR) after 4 cycles of chemotherapy indicative of a higher risk of second-line treatment failure.5
Management of R/R cHL in CAYA must balance efficacy with the risk of late effects of treatment (eg, gonadal dysfunction, cardiac and pulmonary toxicity, and secondary malignancies).5,6 Over 50% of deaths observed among patients aged ≤39 years, who remain without progression for ≥2 years following auto-HCT, are due to nonrelapse mortality (7% at 5 years); notably, 8% of patients have been reported to experience ≥1 nonmalignant late effect.6 New salvage strategies that attain high CMR rates while minimizing long-term toxicity are needed.
Nivolumab, an immune checkpoint inhibitor, is a fully humanized IgG4 anti-programmed death-1 monoclonal antibody; it has demonstrated frequent and durable responses with a favorable safety profile as monotherapy in adults with R/R cHL.7 Brentuximab vedotin (BV), an anti-CD30 antibody conjugated to monomethyl auristatin E, induces apoptosis of CD30-expressing tumor cells by disrupting the microtubule network and inhibiting cell division.8,9 Bendamustine is a bifunctional mechlorethamine derivative that is structurally similar to alkylating agents and purine analogs, and has demonstrated activity in heavily pretreated adults with R/R cHL.10,11 In pediatric patients with R/R cHL, BV monotherapy has shown a complete response (CR) rate of 33% (overall response rate, 47%) with a favorable safety profile12; BV plus bendamustine has demonstrated high CR rates (74% and 66%, respectively) as first salvage in adult and pediatric patients.13,14 The combination of nivolumab plus BV, in a phase 1 to 2 study, has shown high CMR and objective response rates (67% and 85%, respectively) with an acceptable safety profile as first salvage in adult patients with R/R cHL.15
CheckMate 744 is a phase 2 risk-stratified study in CAYA with R/R cHL that combines targeted immunotherapy with antibody-drug conjugate, with the goal of increasing CMR rate and limiting late toxicities. Herein, we report findings from the primary analysis of the standard-risk (R2) cohort with nivolumab plus BV induction, and response-adapted BV plus bendamustine intensification for patients who did not attain a CMR to nivolumab plus BV. Results from the low-risk cohort, which aimed to describe the 3-year PFS among patients treated without auto-HCT, will be reported separately after a planned analysis when follow-up reaches 36 months.
Materials and methods
Study design and patients
CheckMate 744 (ClinicalTrials.gov: NCT02927769) is an international, open-label, multicenter, risk-stratified, response-adapted phase 2 study in CAYA (aged 5-30 years) with R/R cHL. Eligible patients had measurable disease, documented by pathologic and radiographic criteria, including FDG-PET–avid and bidimensional measurable disease of ≥1.5 cm in the longest axis. Patients had pathologically confirmed cHL (excluding nodular lymphocyte-predominant HL) after failure or nonresponse to first-line therapy (ie, relapsed or refractory disease) and a Karnofsky (for patients aged >16 years) or Lansky (for patients aged ≤16 years) performance status ≥50 at study entry. Prior treatment with checkpoint inhibitors, radiation therapy (RT) within 3 weeks (or chest radiation within 12 weeks), bendamustine, auto-HCT, or allogeneic hematopoietic cell transplantation was not permitted; prior BV was permitted.
Risk stratification was based on various criteria (supplemental Table 1, available on the Blood website), in alignment with published EuroNet–Paediatric Hodgkin Lymphoma (EuroNet-PHL) guidelines.5 Standard risk was defined as having ≥1 of the following: primary refractory disease, high stage at initial diagnosis, short time to relapse, presence of B symptoms or extranodal disease at relapse, extensive disease (where RT was contraindicated at relapse), or relapse in a prior radiation field. Patients not eligible for the standard-risk cohort were eligible for a separate, low-risk cohort (results not reported herein). In analysis of efficacy and safety in subgroups of interest, primary refractory patients were defined as those who never achieved remission or experienced progression <3 months after completion of initial therapy. Pediatric patients were those aged <18 years at the time of study enrollment.
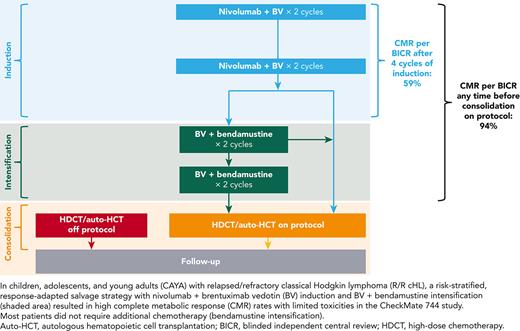
Therapy included induction with nivolumab plus BV for all patients, and intensification with BV plus bendamustine for patients with less than CMR following induction. Patients who attained a CMR at any time following induction or intensification received consolidation with auto-HCT (Figure 1).
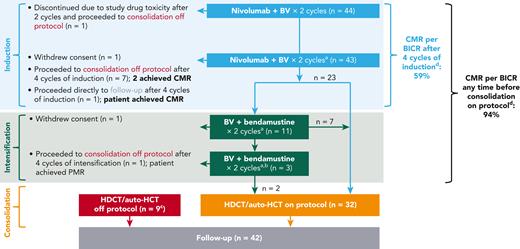
CheckMate 744 R2 cohort study design and patient disposition. Response rates are reported before consolidation. Patients who achieved partial metabolic response (PMR)/no metabolic response (NMR) per blinded independent central review (BICR) after 4 cycles of nivolumab plus BV induction received intensification with BV plus bendamustine. Patients who achieved CMR per BICR after 4 cycles of nivolumab plus BV induction or after 2 or 4 cycles of BV plus bendamustine intensification proceeded to consolidation with high-dose chemotherapy (HDCT) and auto-HCT. Patients who had progressive metabolic disease (PMD) after induction, or PMR, NMR, or PMD after intensification, could proceed to follow-up. Shaded area indicates study treatment phases. aPET–computed tomography/magnetic resonance imaging (real-time BICR) was performed after 4 cycles of nivolumab plus BV and after every 2 cycles of BV plus bendamustine before consolidation. bIf approved by study medical monitor. cOne patient received ifosfamide, carboplatin, and etoposide for progression. dPatients who withdrew consent during the study or who proceeded to follow-up after induction were included for response assessment.
CheckMate 744 R2 cohort study design and patient disposition. Response rates are reported before consolidation. Patients who achieved partial metabolic response (PMR)/no metabolic response (NMR) per blinded independent central review (BICR) after 4 cycles of nivolumab plus BV induction received intensification with BV plus bendamustine. Patients who achieved CMR per BICR after 4 cycles of nivolumab plus BV induction or after 2 or 4 cycles of BV plus bendamustine intensification proceeded to consolidation with high-dose chemotherapy (HDCT) and auto-HCT. Patients who had progressive metabolic disease (PMD) after induction, or PMR, NMR, or PMD after intensification, could proceed to follow-up. Shaded area indicates study treatment phases. aPET–computed tomography/magnetic resonance imaging (real-time BICR) was performed after 4 cycles of nivolumab plus BV and after every 2 cycles of BV plus bendamustine before consolidation. bIf approved by study medical monitor. cOne patient received ifosfamide, carboplatin, and etoposide for progression. dPatients who withdrew consent during the study or who proceeded to follow-up after induction were included for response assessment.
Treatments, end points, and assessments
During induction phase, patients received 4 cycles of nivolumab (3 mg/kg, day 8 of cycle 1; day 1 thereafter) plus BV (1.8 mg/kg, day 1 of every cycle; Figure 1); patients who achieved CMR (Deauville ≤3 per Lugano 2014 criteria3) per blinded independent central review (BICR) after 4 cycles could receive up to 2 additional cycles of nivolumab plus BV if approved by the study medical monitor. Patients who did not achieve CMR per BICR after 4 cycles of induction received intensification with BV plus bendamustine (90 mg/m2, days 1 and 2 of every cycle) for 2 cycles; up to 2 additional cycles of BV plus bendamustine were permitted if approved by the study medical monitor. The goal of this study was to assess CMR before consolidation. Patients who achieved CMR per BICR after induction or intensification proceeded to consolidation with high-dose chemotherapy and auto-HCT, performed per institutional guidelines. As there might have been discrepancy between BICR and investigator assessment, the study also explored outcomes for patients who received treatment outside of the protocol-directed therapy.
The primary end point was CMR rate per BICR any time before consolidation. Secondary end points included objective response rate (ORR) per BICR and investigator after 4 cycles of induction with nivolumab plus BV, CMR rate per investigator any time before consolidation, PFS, duration of response (DOR), and safety of nivolumab plus BV.
Assessment of best reduction in tumor volume was based on the greatest reduction from baseline in the sum of products of diameters for target lesions.
Populations for analysis and statistical considerations
All results were reported on the treated population. Using the Kaplan-Meier product-limit method, PFS was estimated from day 1 of treatment, and DOR was estimated from time of response among response-evaluable patients. For the primary end point, response-evaluable patients were defined as those who achieved CMR per BICR at any time before consolidation, achieved partial metabolic response per BICR at any time, or completed 6 cycles of therapy (4 cycles of nivolumab plus BV and 2 cycles of BV plus bendamustine). Patients who discontinued the study early (including those who discontinued before 4 cycles of induction) due to toxicity without CMR/partial metabolic response were considered response evaluable. For the end point of ORR after 4 cycles, patients were considered response evaluable after 4 cycles of nivolumab plus BV. For CMR and ORR, a 90% confidence interval (CI) was chosen on the basis of the statistical plan and a planned sample size of 40. Safety analyses were performed in all treated patients with 100 days of follow-up; adverse events (AEs) were categorized according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and Medical Dictionary for Regulatory Activities preferred term.
This study was performed in accordance with the Declaration of Helsinki. Approval from the appropriate institutional review board and independent ethics committee was received for the protocol, amendments, and consent forms before initiating the study at each site. This study included children and adolescent patients; for all minors, according to local legislation, parent(s) or legally acceptable representative(s) were informed of the study procedures and signed the informed consent form. Assent was provided for patients aged <18 years, per institution requirements.
Results
Baseline patient characteristics and disposition
In total, 44 patients were treated in the R2 cohort (Table 1). Median age was 16 years, most (70%) were aged <18 years, and approximately half (55%) had primary refractory disease. Sixteen patients (36%) had stage IV disease at relapse. No patient had received prior BV.
Baseline demographics and clinical characteristics
| Characteristic . | R2 cohort (n = 44) . |
|---|---|
| Age, median (range), y | 16 (9-30) |
| <18 y | 31 (70) |
| Male sex | 29 (66) |
| Performance status, median (range) | |
| Lansky, n = 26∗ | 100 (70-100) |
| Karnofsky, n = 18† | 100 (80-100) |
| Stage at initial diagnosis | |
| II | 21 (48) |
| III | 7 (16) |
| IV | 16 (36) |
| Response to first-line therapy | |
| Primary refractory‡ | 24 (55) |
| Relapsed§ | 20 (45) |
| 3-12 mo | 14 (32) |
| ≥12 mo | 6 (14) |
| Prior auto-HCT | 0 |
| Prior BV | 0 |
| Prior systemic therapy | 44 (100) |
| OEPA/COPDAC | 20 (46) |
| OEPA | 6 (14) |
| ABVE-PC | 6 (14) |
| ABV/COPP | 4 (9) |
| ABVD | 4 (9) |
| ABVE | 2 (5) |
| Other | 2 (5) |
| Prior radiation therapy | 16 (36) |
| B symptoms or extranodal disease at relapse, extensive disease (RT contraindicated), or relapse in a prior radiation field | 28 (64) |
| Bone marrow involvement | 5 (11) |
| Stage IV disease at relapse | 16 (36) |
| Characteristic . | R2 cohort (n = 44) . |
|---|---|
| Age, median (range), y | 16 (9-30) |
| <18 y | 31 (70) |
| Male sex | 29 (66) |
| Performance status, median (range) | |
| Lansky, n = 26∗ | 100 (70-100) |
| Karnofsky, n = 18† | 100 (80-100) |
| Stage at initial diagnosis | |
| II | 21 (48) |
| III | 7 (16) |
| IV | 16 (36) |
| Response to first-line therapy | |
| Primary refractory‡ | 24 (55) |
| Relapsed§ | 20 (45) |
| 3-12 mo | 14 (32) |
| ≥12 mo | 6 (14) |
| Prior auto-HCT | 0 |
| Prior BV | 0 |
| Prior systemic therapy | 44 (100) |
| OEPA/COPDAC | 20 (46) |
| OEPA | 6 (14) |
| ABVE-PC | 6 (14) |
| ABV/COPP | 4 (9) |
| ABVD | 4 (9) |
| ABVE | 2 (5) |
| Other | 2 (5) |
| Prior radiation therapy | 16 (36) |
| B symptoms or extranodal disease at relapse, extensive disease (RT contraindicated), or relapse in a prior radiation field | 28 (64) |
| Bone marrow involvement | 5 (11) |
| Stage IV disease at relapse | 16 (36) |
Data are given as number (percentage) unless stated otherwise.
ABV, doxorubicin, bleomycin, and vinblastine; ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; ABVE, doxorubicin, bleomycin, vincristine, and etoposide; ABVE-PC, doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide; COPDAC, cyclophosphamide, vincristine, prednisone, and dacarbazine; COPP, cyclophosphamide, vincristine, procarbazine, and prednisone; OEPA, vincristine, etoposide, prednisone, doxorubicin.
Patients aged ≤16 years.
Patients aged >16 years.
Never achieved remission or who achieved remission to prior therapy, then experienced progression <3 months after completion of that therapy.
Achieved remission to prior therapy, then experienced recurrent disease ≥3 months after completion of that therapy.
At data cutoff, with a minimum potential follow-up of 15.6 months (median observed follow-up, 20.9 months [range, 2.6-29.2 months]), 44 patients completed 2 cycles of induction with nivolumab + BV, and 43 patients completed 4 cycles of induction; 1 patient discontinued after 2 cycles of induction because of toxicity, but was evaluable for response (patient 9 in supplemental Table 2). Of the 43 patients who completed 4 cycles of induction, 23 proceeded to consolidation on protocol and 11 received intensification with BV plus bendamustine (Figure 1). Overall, 41 patients (95%) proceeded to consolidation with auto-HCT: 32 on protocol (23 after completing induction therapy, and 9 patients after intensification) and 9 off protocol at the investigator’s discretion (supplemental Table 2).
Efficacy
The CMR rate per BICR was 59% (26/44) after 4 cycles of induction with nivolumab plus BV, 82% (9/11) after 2 cycles of intensification with BV plus bendamustine, and 94% (33/35) any time before consolidation on protocol (primary end point), with an ORR per BICR of 100% (Table 2). The CMR rate per investigator was 66% (29/44) after 4 cycles of induction with nivolumab plus BV, 91% (10/11) after 2 cycles of intensification with BV plus bendamustine, and 91% (32/35) any time before consolidation on protocol (ORR, 100%). Other CMR and ORR secondary end points are shown in Table 2.
Response rates in response-evaluable patients before consolidation
| Variable . | BICR . | Investigator . |
|---|---|---|
| Induction | ||
| After 2 cycles nivolumab + BV induction | ||
| No. | 44 | 44 |
| ORR | 37 (84) | 38 (86) |
| CMR | 26 (59) | 25 (57) |
| PMR | 11 (25) | 13 (30) |
| NMR | 5 (11) | 2 (5) |
| PMD | 1 (2) | 0 |
| Not evaluable | 0 | 1 (2) |
| Not reported | 1 (2) | 3 (7) |
| After 4 cycles nivolumab + BV induction | ||
| No. | 44 | 44 |
| ORR | 36 (82) | 39 (89) |
| CMR | 26 (59) | 29 (66) |
| PMR | 10 (23) | 10 (23) |
| NMR | 3 (7) | 2 (5) |
| PMD | 1 (2) | 2 (5) |
| Not evaluable | 3 (7) | 0 |
| Not reported | 1 (2)∗ | 1 (2)∗ |
| Intensification | ||
| After 2 cycles BV + bendamustine intensification | ||
| No. | 11 | 11 |
| ORR | 11 (100) | 11 (100) |
| CMR | 9 (82) | 10 (91) |
| PMR | 2 (18) | 1 (9) |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| After 4 cycles BV + bendamustine intensification | ||
| No. | 3 | 2 |
| ORR | 3 (100) | 2 (100) |
| CMR | 2 (67) | 2 (100) |
| PMR | 1 (33) | 0 |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| Any time before consolidation | ||
| Any time before on-protocol consolidation (nivolumab + BV ± BV + bendamustine) | ||
| No. | 35 | 35 |
| ORR | 35 (100) | 35 (100) |
| CMR | 33 (94) | 32 (91) |
| PMR | 2 (6) | 3 (9) |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| Any time before off-protocol consolidation | ||
| No. | 9 | 9 |
| ORR | 7 (78) | 8 (89) |
| CMR | 5 (56) | 7 (78) |
| PMR | 2 (22) | 1 (11) |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| Not evaluable | 1 (11) | 0 |
| Not reported | 1 (11) | 1 (11) |
| Variable . | BICR . | Investigator . |
|---|---|---|
| Induction | ||
| After 2 cycles nivolumab + BV induction | ||
| No. | 44 | 44 |
| ORR | 37 (84) | 38 (86) |
| CMR | 26 (59) | 25 (57) |
| PMR | 11 (25) | 13 (30) |
| NMR | 5 (11) | 2 (5) |
| PMD | 1 (2) | 0 |
| Not evaluable | 0 | 1 (2) |
| Not reported | 1 (2) | 3 (7) |
| After 4 cycles nivolumab + BV induction | ||
| No. | 44 | 44 |
| ORR | 36 (82) | 39 (89) |
| CMR | 26 (59) | 29 (66) |
| PMR | 10 (23) | 10 (23) |
| NMR | 3 (7) | 2 (5) |
| PMD | 1 (2) | 2 (5) |
| Not evaluable | 3 (7) | 0 |
| Not reported | 1 (2)∗ | 1 (2)∗ |
| Intensification | ||
| After 2 cycles BV + bendamustine intensification | ||
| No. | 11 | 11 |
| ORR | 11 (100) | 11 (100) |
| CMR | 9 (82) | 10 (91) |
| PMR | 2 (18) | 1 (9) |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| After 4 cycles BV + bendamustine intensification | ||
| No. | 3 | 2 |
| ORR | 3 (100) | 2 (100) |
| CMR | 2 (67) | 2 (100) |
| PMR | 1 (33) | 0 |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| Any time before consolidation | ||
| Any time before on-protocol consolidation (nivolumab + BV ± BV + bendamustine) | ||
| No. | 35 | 35 |
| ORR | 35 (100) | 35 (100) |
| CMR | 33 (94) | 32 (91) |
| PMR | 2 (6) | 3 (9) |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| Any time before off-protocol consolidation | ||
| No. | 9 | 9 |
| ORR | 7 (78) | 8 (89) |
| CMR | 5 (56) | 7 (78) |
| PMR | 2 (22) | 1 (11) |
| NMR | 0 | 0 |
| PMD | 0 | 0 |
| Not evaluable | 1 (11) | 0 |
| Not reported | 1 (11) | 1 (11) |
Data are given as number (percentage) unless stated otherwise. CMR and PMR rates may not sum to ORR because of rounding. Patients who withdrew consent during the study or who proceeded to follow-up after induction were included for response assessment.
NMR, no metabolic response; PMD, progressive metabolic disease; PMR, partial metabolic response.
One patient discontinued because of study drug toxicity after 2 cycles of induction with nivolumab plus BV.
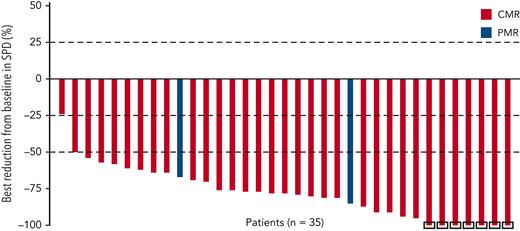
Among 35 patients evaluable for metabolic response per BICR, 34 (97%) achieved a ≥25% reduction in tumor volume (Figure 2); all patients achieved a ≥25% reduction in maximum standardized uptake value (supplemental Figure 1), and 33 patients (94%) achieved a ≥50% reduction. Median DOR per BICR was not reached.
Best reduction in tumor volume per BICR in patients who proceeded to consolidation on protocol. On the basis of sum of products of diameters (SPD) for target lesions. Includes patients with baseline and ≥1 on-study value (before consolidation or subsequent therapy). Negative values indicate reduction from baseline in SPD. Square symbol represents percentage change truncated to 100%. PMR, partial metabolic response.
Best reduction in tumor volume per BICR in patients who proceeded to consolidation on protocol. On the basis of sum of products of diameters (SPD) for target lesions. Includes patients with baseline and ≥1 on-study value (before consolidation or subsequent therapy). Negative values indicate reduction from baseline in SPD. Square symbol represents percentage change truncated to 100%. PMR, partial metabolic response.
Among response-evaluable patients with primary refractory disease (n = 23), at any time before consolidation, 20 (87%) achieved CMR (90% CI, 70%-96%) per BICR, and 22 (96%) achieved an objective response per BICR; 15 (63%) achieved CMR per BICR after 4 cycles of induction. Similarly, among response-evaluable pediatric patients (n = 30), at any time before consolidation, 27 (90%) achieved CMR (90% CI, 76%-97%) per BICR, and all patients achieved an objective response per BICR; 18 (58%) achieved CMR per BICR after 4 cycles of induction.
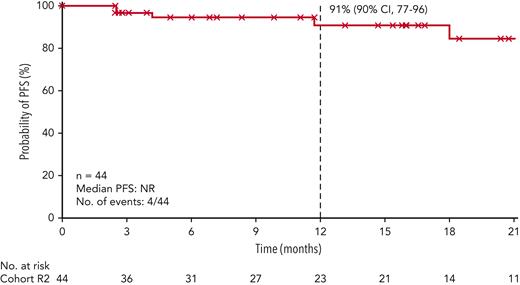
At data cutoff, median PFS per BICR among all treated patients had not been reached; the 12-month PFS rate was 91% (90% CI, 77%-96%; Figure 3). Four patients experienced relapse after achieving a CMR (1 after induction and 3 after intensification). Median overall survival in all treated patients was not reached.
PFS per BICR. Symbols represent censored observations. Patients who did not progress or die, or who started subsequent therapy (including auto-HCT off protocol) without prior reported progression or death, were censored at the last tumor assessment. NR, not reached.
PFS per BICR. Symbols represent censored observations. Patients who did not progress or die, or who started subsequent therapy (including auto-HCT off protocol) without prior reported progression or death, were censored at the last tumor assessment. NR, not reached.
For the 9 patients who proceeded to consolidation off protocol, 5 achieved CMR per BICR and 7 achieved CMR per investigator any time before consolidation (Table 2). Detailed response outcomes are shown in supplemental Table 2. Of these 9 patients, 3 received off-protocol treatment before auto-HCT. These patients were not included in the final response evaluation.
Off-protocol therapies and auto-HCT
Post–auto-HCT therapies
Among all patients, 27 (61%) received any subsequent therapy. The most common reason for subsequent systemic therapy was maintenance without disease progression or clinical deterioration (supplemental Table 3). The most common subsequent systemic therapy was BV (13 patients [30%]). Ten patients received BV after auto-HCT as maintenance, 8 received RT as consolidation after auto-HCT, and 1 received both BV and RT.
Off-protocol consolidation and auto-HCT
All 9 patients who proceeded to off-protocol consolidation received auto-HCT (supplemental Table 2).
Off-protocol consolidation and subsequent therapies
Three patients received chemotherapy before consolidation off protocol (supplemental Table 2): 1 received gemcitabine, ifosfamide, prednisolone, and vinorelbine; 1 received ifosfamide, carboplatin, and etoposide; and 1 received bendamustine. One patient did not proceed to consolidation after induction but proceeded directly to follow-up; this patient experienced progression and had undergone surgery and received BV, cisplatin, cytarabine, dexamethasone, BEAM (carmustine, etoposide, cytarabine, and melphalan), and RT (curative) as subsequent therapies.
Stem cell mobilization
In patients who received subsequent auto-HCT, the most commonly used stem cell mobilization agent was granulocyte colony stimulating factor or a recombinant form thereof (22 of 40 patients [55%] for whom mobilization was reported; supplemental Table 4). The most common conditioning regimen prior to auto-HCT was BEAM (23/44 patients [52%]). Each patient had a median of 1 (range, 1-5) apheresis session; a median of 4.0 (range, 0.3-268.0) × 106 CD34+ cells/kg were collected per session.
Safety
Overall, 31 patients (70%) experienced a treatment-related AE (TRAE) during nivolumab plus BV induction, including grade 3/4 TRAEs in 18% of patients (Table 3). The most common TRAEs during induction were hypersensitivity (20%), nausea (20%), and diarrhea (14%) (all grades 1/2). Of the 11 patients who required intensification with BV plus bendamustine, 8 (73%) experienced a TRAE (grade 3/4 in 3 [27%] patients). Treatment-related serious AEs (SAEs) occurred in 5 patients (11%) before consolidation (grade 3/4 in 3 patients). One TRAE led to discontinuation (grade 3 anaphylactic reaction). There were no treatment-related deaths; 1 patient died because of disease progression.
Patients with TRAEs before consolidation and treatment-related IMAEs
| Variable . | Induction (n = 44) . | Intensification (n = 11) . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Any TRAE | 31 (70.5) | 8 (18.2)∗ | 8 (72.7) | 3 (27.3)† |
| TRAEs‡with ≥2 patients in either phase | ||||
| Hypersensitivity | 9 (20.5) | 0 | 1 (9.1) | 0 |
| Nausea | 9 (20.5) | 0 | 5 (45.5) | 0 |
| Diarrhea | 6 (13.6) | 0 | 1 (9.1) | 0 |
| Infusion-related reaction | 5 (11.4) | 1 (2.3) | 2 (18.2) | 0 |
| Abdominal pain | 4 (9.1) | 0 | 0 | 0 |
| Pyrexia | 4 (9.1) | 0 | 1 (9.1) | 0 |
| Rash | 4 (9.1) | 0 | 0 | 0 |
| Maculopapular rash | 3 (6.8) | 0 | 0 | 0 |
| Vomiting | 3 (6.8) | 0 | 6 (54.5) | 1 (9.1) |
| Alopecia | 2 (4.5) | 0 | 0 | 0 |
| Arthralgia | 2 (4.5) | 0 | 0 | 0 |
| Fatigue | 2 (4.5) | 0 | 0 | 0 |
| Increased AST | 2 (4.5) | 0 | 1 (9.1) | 0 |
| Pruritus | 2 (4.5) | 0 | 0 | 0 |
| Upper abdominal pain | 2 (4.5) | 0 | 0 | 0 |
| Headache | 1 (2.3) | 0 | 2 (18.2) | 0 |
| Variable . | Induction (n = 44) . | Intensification (n = 11) . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Any TRAE | 31 (70.5) | 8 (18.2)∗ | 8 (72.7) | 3 (27.3)† |
| TRAEs‡with ≥2 patients in either phase | ||||
| Hypersensitivity | 9 (20.5) | 0 | 1 (9.1) | 0 |
| Nausea | 9 (20.5) | 0 | 5 (45.5) | 0 |
| Diarrhea | 6 (13.6) | 0 | 1 (9.1) | 0 |
| Infusion-related reaction | 5 (11.4) | 1 (2.3) | 2 (18.2) | 0 |
| Abdominal pain | 4 (9.1) | 0 | 0 | 0 |
| Pyrexia | 4 (9.1) | 0 | 1 (9.1) | 0 |
| Rash | 4 (9.1) | 0 | 0 | 0 |
| Maculopapular rash | 3 (6.8) | 0 | 0 | 0 |
| Vomiting | 3 (6.8) | 0 | 6 (54.5) | 1 (9.1) |
| Alopecia | 2 (4.5) | 0 | 0 | 0 |
| Arthralgia | 2 (4.5) | 0 | 0 | 0 |
| Fatigue | 2 (4.5) | 0 | 0 | 0 |
| Increased AST | 2 (4.5) | 0 | 1 (9.1) | 0 |
| Pruritus | 2 (4.5) | 0 | 0 | 0 |
| Upper abdominal pain | 2 (4.5) | 0 | 0 | 0 |
| Headache | 1 (2.3) | 0 | 2 (18.2) | 0 |
| Treatment-related IMAE category . | Overall (n = 44) . | Pediatric (n = 31) . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Rash§ | 4 (9) | 0 | 1 (3) | 0 |
| Immune-mediated IRR | 3 (7) | 1 (2) | 2 (6) | 0 |
| Hypersensitivity | 2 (5) | 0 | 2 (6) | 0 |
| Hypothyroidism | 1 (2) | 0 | 1 (3) | 0 |
| Pneumonitis | 1 (2) | 0 | 0 | 0 |
| Treatment-related IMAE category . | Overall (n = 44) . | Pediatric (n = 31) . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Rash§ | 4 (9) | 0 | 1 (3) | 0 |
| Immune-mediated IRR | 3 (7) | 1 (2) | 2 (6) | 0 |
| Hypersensitivity | 2 (5) | 0 | 2 (6) | 0 |
| Hypothyroidism | 1 (2) | 0 | 1 (3) | 0 |
| Pneumonitis | 1 (2) | 0 | 0 | 0 |
Data are given as number (percentage).
AST, aspartate aminotransferase; IMAE, immune-mediated adverse event; IRR, infusion-related reaction.
Included anaphylactic reaction, prolonged activated partial thromboplastin time, increased amylase, increased lipase, decreased neutrophil count, neutropenia, hepatotoxicity, and jugular vein thrombosis (n = 1 each).
One patient experienced vomiting and decreased white blood cell count; one experienced increased lipase; and one experienced neutropenia.
As Common Terminology Criteria for Adverse Events preferred terms.
Included rash, maculopapular rash, and pruritic rash.
Among treated pediatric patients (n = 31), 23 (74%) experienced a TRAE during induction (grade 3/4, 19%). The most common TRAEs in pediatric patients during induction were nausea (n = 8, 26%), hypersensitivity (n = 7, 23%), and diarrhea (n =5, 16%). In patients with refractory disease (n = 24), 18 (75%) experienced a TRAE during induction (grade 3/4, 21%).
During induction, 8 of 44 patients (18%) were hospitalized because of AEs, 1 each due to treatment-related SAEs of grade 3 fever and infusion-related reaction (IRR), grade 2 allergic reaction, and grade 3 anaphylactic reaction. All were discharged within 48 hours. The remaining 5 patients (11%) were hospitalized for SAEs unrelated to treatment; no hospitalizations were reported for drug administration.
Treatment-related neutropenia (grade 3) was reported in 1 patient during induction and in 1 patient during intensification; grade 3 treatment-related decreased neutrophil count was reported in 1 patient during induction, and grade 3 decreased white blood cell count was reported in 1 patient during intensification. There were no other treatment-related hematologic AEs. Treatment-related peripheral sensory neuropathy was reported in 1 patient during induction (grade 1).
Overall, treatment-related immune-mediated AEs (IMAEs) were primarily grade 1/2, and 1 patient experienced grade 3/4 immune-mediated IRR (Table 3). Six treatment-related IMAEs were reported in pediatric patients; all were grade 1/2 and not dose limiting. All reported IMAEs, except for hypothyroidism, required treatment with corticosteroids and were resolved.
IRRs were reported in 8 patients during induction (6 grade 1/2 and 2 grade 3) and in 2 patients during intensification (both grade 1/2). Most IRRs occurred during induction cycle 2 (15 events).
Discussion
The CheckMate 744 study evaluates first salvage therapy in one of the largest cohorts of pediatric patients with cHL using a checkpoint inhibitor, and demonstrated the highest CMR rates in this population.12,16 It is also the first international collaboration between the Children’s Oncology Group (COG) and the EuroNet-PHL in relapsed pediatric HL. The safety and efficacy of the regimen described in this study supports checkpoint inhibitors as first salvage for this population with a curative intent. CAYA with R/R cHL who achieved a CMR to therapy before auto-HCT have been shown to have better outcomes than those without CMR.2,3 The use of a risk-stratified, response-adapted approach with nivolumab plus BV induction, and BV plus bendamustine intensification in patients with suboptimal response, resulted in a CMR rate of 94% per BICR before consolidation, and 59% after just 4 cycles of induction without conventional chemotherapy. Just 11 of 44 patients required intensification with BV plus bendamustine, and most treated patients (95%) proceeded to consolidation with auto-HCT. Although 9 patients received consolidation off protocol, the induction/intensification therapy received was consistent with the study protocol.
As most of the study population was pediatric patients (n = 30/44) and the CMR rate per BICR any time before consolidation in this subgroup was 90% (overall, 94%), the CMR rate in patients aged 18 to 30 years was expected to be similar to that in the pediatric subgroup. Also, the CMR rate after 4 cycles of nivolumab plus BV induction in these young adults (overall, 59%) was expected to be comparable to the CR rates previously reported for the adult population treated with 4 cycles of nivolumab and BV (67%).15 The high CMR rates observed with this regimen were also seen in patients with primary refractory disease (CMR per BICR, 87%), who comprised 55% of patients in this study, making this regimen a viable option for these high-risk patients. Notable proportions of patients also had high-risk clinical features at study entry, including stage IV disease (36%), B symptoms or extranodal involvement (64%), and bone marrow involvement (11%).
There were slight discrepancies between response rates per BICR and per investigator assessment, which could be attributed to the reports of SAEs that may skew the PET–computed tomography results. During the study, 1 patient experienced herpes zoster, 4 experienced non–drug-related pericardial effusion, and 4 experienced pneumonia (1 atypical and 3 organizing events). These clinical details were not disclosed as per BICR, which impacted the interpretation of the FDG-PET responses in these selected patients.
The response-adapted approach aimed to limit the risk of long-term toxicity. Nivolumab plus BV induction was well tolerated, with few treatment-related hematologic toxicities or AEs leading to discontinuation. Treatment-related IMAEs also had a low incidence and severity during the induction phase, suggesting autoimmune toxicities had minimal impact on therapy. A greater proportion of patients experienced grade 3/4 TRAEs (27%) during intensification than during induction (18%). The favorable safety profile was also observed among pediatric patients, who comprised 70% of the study population. The high CMR rates after induction, combined with the favorable safety profile of nivolumab plus BV, suggest that intensification with BV plus bendamustine could be safely reserved for a subset of patients with an inadequate response to induction therapy.
Numerous first salvage strategies for CAYA with cHL have been developed, but there is no standard of care. Recent studies have generally focused on treatment intensification for achieving remission while limiting short-term toxicities and late effects.17-23 Encouraging results have been shown in young patients using BV in combination with conventional chemotherapy, although cross-trial comparisons should be made with caution because of differences in patient populations and procedures. In the AHOD1221 trial, BV plus gemcitabine demonstrated a CR rate of 67% in CAYA, but was associated with a relatively high frequency of grade 4 neutropenia (36%).17 BV plus ifosfamide, gemcitabine, and vinorelbine has shown a CMR rate of 71% as first or subsequent salvage in a study of 28 young adult patients, although the rates of grade 3/4 hematologic toxicity (96% neutropenia and 89% thrombocytopenia) and any-grade febrile neutropenia (57%) were also high.18 Initial data in adult patients suggest that BV may also be successfully combined with dexamethasone, high-dose cytarabine, and cisplatin; longer follow-up from the phase 2 expansion cohort in that study showed a high CMR of 79%.19,24 Other PET-adapted sequential therapy strategies have also shown promise, including BV monotherapy followed by BV plus augmented ifosfamide, carboplatin, and etoposide. The CMR rate, as defined in that study (Deauville 1-2) after BV monotherapy, was 27%, and 44% if defining CMR using a Deauville score of 1 to 3.20 Etoposide-based therapies have been associated with risks of secondary malignancies and infertility (mainly due to combination with procarbazine),25,26 as well as therapy-related myelodysplasia and acute myeloid leukemia after transplantation.27
Notably, most of these studies include chemotherapy as part of either the regimen backbone or a response-adapted strategy. The present study design was built on the salvage strategy (ie, salvage therapy followed by auto-HCT) used in previous trials conducted by the COG,17 but relatively high CMR rates were attained without conventional chemotherapy, and just 11 patients required intensification with BV plus bendamustine; most patients achieved CMR within 2 cycles of intensification, and just 3 received >2 cycles. The avoidance of conventional chemotherapy in this study resulted in a relatively low incidence of treatment-related hematologic toxicity compared with the high rates reported previously for other cHL salvage regimens.17,18 Furthermore, just 3 of 44 patients were hospitalized because of AEs related to the regimen, suggesting that the regimen appeared to be suitable for administration in the outpatient setting.
Relevant to any treatment delivered before auto-HCT, stem cell mobilization and collection proved feasible during this salvage regimen. In this study, a median of 1 apheresis session was required per patient, and collection of CD34+ cells was possible in most patients.
Treatment given after auto-HCT was not defined by the study and relied on investigator choice. Indeed, 27 of 44 patients received subsequent therapies, 10 with BV maintenance and 9 with RT (supplemental Table 3). BV maintenance has been shown to have a sustained PFS benefit in the AETHERA study, which enrolled BV-naive patients with a high risk of relapse after auto-HCT.28 RT delivered before or after auto-HCT has been shown to improve PFS in patients with R/R cHL in retrospective studies.4,29
Although risk stratification for first salvage in CAYA with cHL is evolving, the risk stratification for CheckMate 744 is generally consistent with published EuroNet-PHL guidelines.5 These guidelines combine features such as time to relapse, prior first-line treatment (including extent of RT), and stage at relapse as major prognostic factors. Results from the low-risk cohort in this trial will be published separately.
Limitations of this study were the small sample size and duration of minimum follow-up of <18 months, precluding detection of late or rare events. However, the sample size was determined according to the primary end point for the R2 cohort, and is similar to other phase 2 trials in CAYA.17,22 As no patients in this study had prior exposure to BV, the efficacy of the regimen used in this study in CAYA with prior BV exposure is unknown. The heterogeneity of off-protocol therapy was a confounder to interpreting PFS outcomes following auto-HCT; however, this was not the primary end point of the study. Although the use of BV could possibly delay progression or death,30 just 10 patients received maintenance with BV; thus, the impact of BV maintenance on PFS is believed to be minimal. Because of the small sample size and number of events, PFS was not analyzed in the subgroups of patients who received consolidation off protocol and/or subsequent therapy. Subsequent therapies may limit the interpretation of overall survival. Unlike alkylating agents and anthracyclines, the long-term effects of checkpoint inhibitors in pediatric patients are not yet known, although data from adult melanoma studies suggest that long-term toxicities are limited.31 The highly active induction phase based on targeted therapies in this study limited exposure to bendamustine chemotherapy and may have reduced the incidence of late effects compared with previously reported regimens.
In conclusion, this first risk-adapted, response-based approach resulted in some of the highest rates of CMR in recent studies conducted cooperatively by pediatric groups for CAYA with R/R cHL. The notable CMR rates after induction with nivolumab plus BV suggest that intensification with BV plus bendamustine could be safely reserved for a subset of patients with an inadequate response to induction therapy, thereby eliminating additional exposure to alkylators for most patients. CheckMate 744 is the first collaboration between the COG and EuroNet-PHL for CAYA with R/R HL, and as the largest pediatric trial using a checkpoint inhibitor, it has demonstrated that this treatment modality is an efficacious and well tolerated first salvage for this population. Further follow-up will help to assess the durability of disease control and long-term safety of this regimen combined with auto-HCT. Future trials could also evaluate the efficacy and safety of this regimen in first-line treatment in CAYA.
Acknowledgments
The authors thank Catherine Curtillet for her help in patient enrollment in this study, as well as the patients, their families, and all coinvestigators who participated in the trial.
Professional medical writing and editorial support were provided by Adam Gill and Jane Cheung, Caudex, funded by Bristol Myers Squibb. This work was supported by Bristol Myers Squibb, in collaboration with Seagen. Direct funding was provided by Bristol Myers Squibb through the joint financial support of Bristol Myers Squibb and Seagen.
The views expressed in this article are the authors’ own and not an official position of Bristol Myers Squibb or the authors’ respective institutions.
Authorship
Contribution: R.A.D., T.L., B.S.H., P.D.C., K.M.K., P.H.-M., S.D., C.M.-K., and A.B. conceived and designed the study; C.M.-K., T.L., A.B., K.M.K., and M.M. provided study materials or patients; R.A.D., C.M.-K., P.D.C., and K.M.K. collected and assembled data; R.A.D., C.M.-K., T.L., P.D.C., and K.M.K. performed data analysis and interpretation; all authors wrote the manuscript; all authors provided final approval of the manuscript; and all authors are accountable for all aspects of the work.
Conflict-of-interest disclosure: C.M.-K. received research grants, payment/honoraria for lectures, presentations, and payment for participation on a data safety monitoring board from Merck Sharp and Dohme; and is the Scientific Secretary (unpaid) of the EuroNet–Paediatric Hodgkin Lymphoma Consortium. T.L. received payment for travel and accommodation from Bristol Myers Squibb. G.M. received payment for participation in the study protocol from Bristol Myers Squibb. S.C. received payment/honoraria from Jazz Pharmaceuticals and Pfizer for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events, and payment from Covington LLC for expert testimony. K.J.L. received research grants from Abbott Laboratories, consulting fees from Jazz Pharmaceuticals, and payment from BTG and Jazz Pharmaceuticals for participation on an advisory board. B.S.H. received funding from Merck via the Children’s Oncology Group for participation on a data safety monitoring board/advisory board. J.L. has equity interest and is an employee of Seagen, Inc. S.F. has stock options and was an employee of Bristol Myers Squibb. M.S. was an employee of Bristol Myers Squibb. K.M.K. received payment from Merck via the Children’s Oncology Group for participation on a study steering committee and is a member on the scientific advisory board (unpaid) for Lymphoma Research Foundation. The remaining authors declare no competing financial interests.
Correspondence: Stephen Daw, Paediatric Division, University College Hospital, 250 Euston Rd, London NW1 2PG, United Kingdom; e-mail: stephendaw@nhs.net.
References
Author notes
∗P.H.-M. and C.M.-K. contributed equally to this study.
†K.M.K. and S.D. contributed equally to this study.
Interim analyses of CheckMate 744 were presented at the 60th American Society of Hematology Annual Meeting, San Diego, CA, 1 to 4 December 2018; the 24th Congress of the European Hematology Association, Amsterdam, The Netherlands, 13 to 16 June 2019; and the 15th International Conference on Malignant Lymphoma, Lugano, Switzerland, 18 to 23 June 2019. Results from this primary analysis have been presented at the American Society of Clinical Oncology 2020 Virtual Scientific Program, 29 to 31 May 2020; and the 25th Congress of the European Hematology Association, Virtual, 9 to 17 June 2020.
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal