Key Points
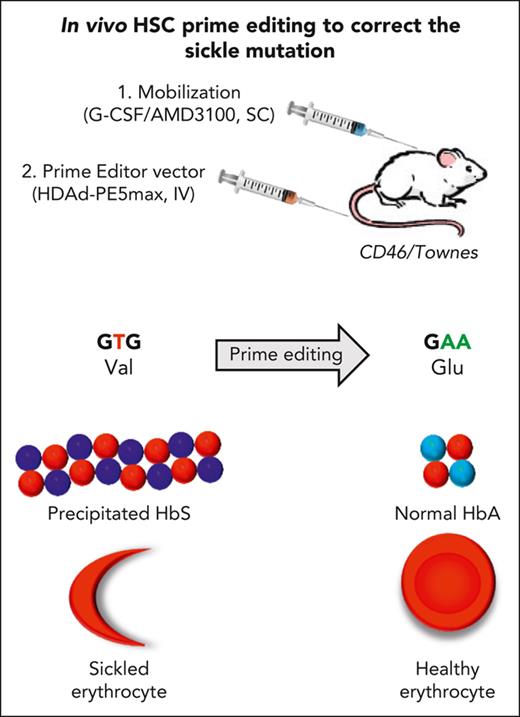
Correction of the sickle-cell mutation and disease phenotypes is achieved by in vivo HSC transduction with vectorized prime editors.
Our approach for in vivo HSC prime editing that does not require HSC transplantation and myeloablation should simplify HSC gene therapy.
Abstract
Sickle cell disease (SCD) is a monogenic disease caused by a nucleotide mutation in the β-globin gene. Current gene therapy studies are mainly focused on lentiviral vector–mediated gene addition or CRISPR/Cas9–mediated fetal globin reactivation, leaving the root cause unfixed. We developed a vectorized prime editing system that can directly repair the SCD mutation in hematopoietic stem cells (HSCs) in vivo in a SCD mouse model (CD46/Townes mice). Our approach involved a single intravenous injection of a nonintegrating, prime editor–expressing viral vector into mobilized CD46/Townes mice and low-dose drug selection in vivo. This procedure resulted in the correction of ∼40% of βS alleles in HSCs. On average, 43% of sickle hemoglobin was replaced by adult hemoglobin, thereby greatly mitigating the SCD phenotypes. Transplantation in secondary recipients demonstrated that long-term repopulating HSCs were edited. Highly efficient target site editing was achieved with minimal generation of insertions and deletions and no detectable off-target editing. Because of its simplicity and portability, our in vivo prime editing approach has the potential for application in resource-poor countries where SCD is prevalent.
Introduction
Sickle cell disease (SCD) is caused by mutations in the HBB gene, which encodes the β-globin subunit of adult hemoglobin (HbA, α2β2).1 Most affected individuals are homozygous for a Glu6Val substitution resulting in the production of βS-globin, which complexes with α-globin to form sickle Hb (HbS) (α2βS2). Under hypoxic conditions, HbS forms rigid polymers that cause red blood cells (RBCs) to acquire a sickle shape and initiate a complex pathophysiology. Preventing the polymerization of HbS can avert disease symptoms. Several HSC gene therapy approaches for SCD are currently being evaluated clinically involving (1) gene addition of antisickling β-globin variants or fetal γ-globin, which also has powerful antipolymerization properties,2 or (2) the reactivation of fetal γ-globin by blocking repressive mechanisms mediated by transcription factors ZBTB7A and BCL11A.3,4 These approaches result in the formation of tetramers of 2 α-globin chains with 1 or 2 antisickling globin chains, with βS-globin chain being largely excluded from the polymer.2 Clinical trials showed impressive therapeutic effects in patients with SCD, specifically reduced hemolysis and complete resolution of severe vaso-occlusive events. These strategies are, however, complex, which involve harvesting HSCs through leukapheresis or bone marrow (BM) aspiration, myeloablation by chemotherapy, in vitro HSC culture, and transplantation.
Notably, expression or reactivation of antisickling globins does not eliminate HbS, which can compete for incorporation into the tetramer and limit the therapeutic efficacy. The most desirable strategy for genetic correction of SCD is to convert the pathogenic codon (valine, GTG) to the wild-type (glutamic acid, GAG). Recently developed prime editors (PEs) can catalyze the T>A conversion. PEs contain a catalytically impaired SpCas9 nickase (nCas9) fused to an engineered reverse transcriptase (RT) that directly copies edited sequence information from a prime editing guide RNA (pegRNA) into a target DNA locus. This causes the cell to replace the original DNA sequence on both strands with the newly synthesized DNA flap. Because the PE system involves 3 separate DNA binding events between (1) the guide sequence and the target DNA, (2) the primer binding site and the target DNA, and (3) the 3’ end of the nicked DNA strand and the pegRNA, it has been suggested to have fewer undesirable off-target (OT) effects than CRISPR/Cas9.5-7 Furthermore, in contrast to CRISPR/Cas9, PEs are independent of double-strand DNA breaks, therefore minimizing the generation of unwanted random insertions and deletions (indels) at the target site and large genomic rearrangements or translocations.8 Recent improvement in the PE architecture,9 the pegRNAs,10 and the inclusion of an engineered dominant-negative MLH1 gene (eg, PE5max) resulted in efficient target site editing and fewer undesired indels.
The multicomponent structure of PE5max would require that the gene transfer vector has an insert capacity of at least 16 kb. Current lentiviral and recombinant adeno-associated virus (rAAV) vectors can only carry payloads of 4.5 and 8 kb, respectively.11,12 We, therefore, used helper-dependent adenoviral (HDAd) vectors for in vivo prime editing. HDAd vectors (1) have a payload capacity of 35 kb; (2) are targeted to HSCs through their fiber protein13; (3) avoid transduction of nonhematopoietic tissues (including hepatocytes); (4) can transduce nondividing cells; (5) remain episomal, allowing for transient expression of genome editors14,15; and (6) can be produced easily at low costs at very high yields and titers because, unlike rAAV and lentivectors, no large-scale plasmid transfection is required. Here, we used HDAd5/35++ vectors with high affinity toward CD46, a receptor that is uniformly expressed on primitive HSCs.13 This enables efficient HSC transduction at low vector doses.
Another major advantage of using the HDAd vector approach is the ability to use an in vivo approach for prime editing. Without HSC mobilization, direct transduction of BM HSCs with intravenously injected HDAd5/35++ vectors is inefficient because of physical barriers formed by the BM stroma.13 Therefore, our approach involved subcutaneous injections of granulocyte-colony stimulating factor (G-CSF)/plerixafor (AMD3100) to mobilize HSCs from the BM into the peripheral blood (PB) stream, followed by an intravenous injection of the HDAd5/35++-PE vector. A large fraction of transduced HSCs returned to the BM. To expand in vivo–transduced HSCs, we are currently using an in vivo selection mechanism based on a mutant O6-methylguanine DNA methyltransferase (MGMTP140K) gene that confers resistance to O6-BG/BCNU (O6-Benzylguanine/Carmustine) given at doses that are lower than those used for cancer chemotherapy.16-18 In ex vivo HSC gene therapy studies, this approach allowed for stable multilineage in vivo selection in mice, dogs, nonhuman primates, and humans without signs of myeloablation.18-20 We have shown that in vivo HSC transduction in mobilized mice resulted in therapeutic efficacy in several mouse disease models.21-24 First data in nonhuman primates show that the in vivo HSC gene therapy approach is safe when combined with glucocorticoid, interleukin 6 (IL-6)- and IL-1β–receptor antagonist pretreatment to suppress innate immune responses after IV HDAd5/35++ injection.25,26 Advantages and disadvantages of ex vivo and in vivo HSC gene therapy approaches are listed in supplemental Table 1; available on the Blood website.
Here, we used HDAd5/35++ vectors to deliver PEs to HSCs to correct the SCD mutation in patient HSCs and in a SCD mouse model (Townes). In Townes mice the murine α-globin genes were replaced with the human α-globin genes; the murine β-globin genes were replaced with human sickle βS and fetal γ-globin genes linked together.27 Townes mice exhibit many of the symptoms of SCD, including short RBC half-life, RBC sickling, high PB reticulocyte frequencies, and splenomegaly. For our studies, Townes mice were crossed with human CD46–transgenic mice to allow for HDAd5/35++ transduction via CD46. We achieved therapeutic levels of prime editing in HSCs of patients with SCD and in CD46/Townes mice.
Material and methods
Detailed information about the following methods is provided in the supplemental Material: reagents for in vivo transduction and selection, construction of HDAd-PE vectors, cell culture, in vitro erythroid differentiation (ED) of CD34+ cells with O6BG/BCNU selection, MLH1 western blot analysis, analysis of reactive oxygen species (ROS) levels, cytospin slide preparation, colony–forming unit (CFU) assay, animal studies, tissue analyses, blood analyses, measurement of gene editing using Sanger sequencing and next-generation sequencing (NGS), measurement of vector copy number, flow cytometry, hemoglobin high-performance liquid chromatography, hemoglobin electrophoresis through isoelectric focusing, detection of hemoglobin subunits using mass spectrometry, OT analysis using CIRCLE-seq and Cas-OFFinder, inverse polymerase chain reaction analysis. Details of CIRCLE-seq and Cas-OFFinder nominated OT sites in human and mouse genomes are provided in supplemental Tables 2-4. Information regarding the oligos used for cloning are listed in supplemental Table 5.
CD34+ cells from healthy donors and patients with SCD
The Fred Hutch Cell Processing Facility provided the CD34+ cells from G-CSF–mobilized healthy adult donors. CD34+ cells from patients with SCD (βS/β0 [n = 1] or βS/βS [n = 2]) were immunomagnetically isolated from exchange transfusion blood samples at the G. Papanikolaou Hospital (written informed consent was obtained from all patients, according to the local research ethics committee). PB mononuclear cells (PBMCs) were isolated from PB using Ficoll density gradient centrifugation followed first by depletion of CD235a+ cells (a considerable percentage of SCD blood cells coexpress CD34 and CD235A owing to stress erythropoiesis28 and then by CD34 positive selection (Miltenyi Biotec). A range between 1.8 × 105 and 3.6 × 105 of CD34+ cells were isolated from ∼320 to 350 mL of steady-state blood. CD34+ cells were incubated overnight in StemSpan H3000 medium (STEMCELL Technologies, Vancouver, Canada) supplemented with penicillin/streptomycin, Flt3 ligand (Flt3L, 25 ng/mL), IL-3 (10 ng/mL), thrombopoietin (2 ng/mL), and stem cell factor (25 ng/mL). Cytokines and growth factors were obtained from Peprotech (Rocky Hill, NJ). CD34+ cells were transduced with HDAd5/35++ vectors in low-attachment 12-well plates.
Animal studies
All experiments involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. University of Washington Institutional Animal Care and Use Committee (protocol number 3108-01) approved the studies. C57BL/6 based transgenic mice that contained the human CD46 genomic locus and provided CD46 expression at a level and in a pattern similar to humans (hCD46+/+ mice) were described earlier.29 A Townes male mouse (Hbbtm2(HBG1,HBB∗)Tow or hα/hα::βS/βS) was purchased from the Jackson Laboratory (JAX stock #013071) and bred with human CD46 transgenic female mice. After 3 rounds of breeding, mice homozygous for CD46, HBBS and HBA were obtained and used for experiments.
Statistical analyses
Statistical significance was calculated by appropriate statistical tests as described in the figure legends. Statistical analysis was computed on GraphPad Prism version 9.0.0 (GraphPad Software Inc, La Jolla, CA). P < .05 was considered as statistically significant.
Results
HDAd vectors expressing PEs
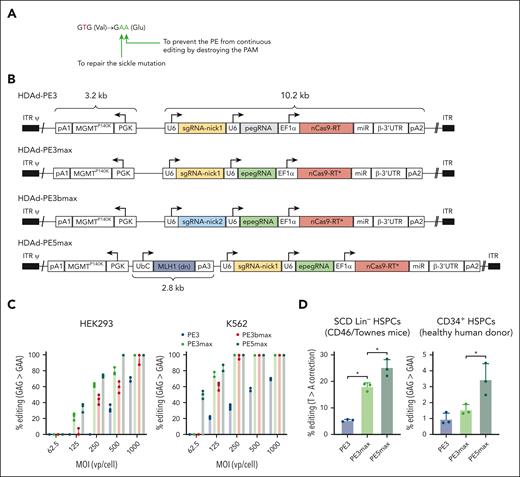
We developed a prime editing system that can directly repair the A>T sickle mutation in HSCs after in vivo transduction with HDAd5/35++ vector. We designed and produced a panel of HDAd vectors expressing various versions of PEs (PE3, PE3max, PE3bmax, and PE5max). HDAd-PE3 consists of a pegRNA mapped to the sickle mutation site, a nicking single guide RNA (sgRNA) to nick the nonedited strand, and a nCas9 linked to an M-MLV RT which is responsible for DNA synthesis according to the RT template contained in the pegRNA to repair the sickle mutation (Figure 1A-B; supplemental Figure 1). In addition to fixing the sickle mutation (T>A conversion), a G>A silent mutation is introduced to prevent the PE from continuing editing by destroying the protospacer-adjacent motif (PAM) (Figure 1A). HDAd-PE3max features an engineered pegRNA (epegRNA) containing a protective motif and an architecture-optimized nCas9-RT. In previous studies, both features were shown to improve the editing efficiency.9,10 HDAd-PE3bmax differs from HDAd-PE3max in the nicking sgRNA, which only matches with PAM-containing DNA strands (after editing takes place) and results in improved product purity. HDAd-PE5max is equivalent to HDAd-PE3max plus a dominant-negative MLH1 cassette, which limits cellular mismatch repair responses and results in increased prime editing efficiency.9 An MGMTP140K expression cassette was included in all vectors for selection of transduced hematopoietic stem and progenitor cells (HSPCs).16 The total transgene size was ∼16 kb.
HDAd vectors expressing prime editors and in vitro validation. (A) Intended base conversions. The goal is to convert the GTG (Val) codon to a GAA (Glu) codon to repair the sickle mutation (T>A) and to prevent the PE from continuing editing by destroying the PAM (G>A silent mutation). (B) Schematics of HDAd vectors used in this study. The prime editing machinery consists of (1) a prime editing guide RNA (pegRNA), capable of identifying the target nucleotide sequence to be edited and encoding new genetic information that replaces the targeted sequence. The pegRNA consists of an extended single guide RNA (sgRNA) containing a primer binding site (PBS) and a reverse transcriptase (RT) template sequence. During genome editing, the primer binding site allows the 3’-end of the nicked DNA strand to hybridize to the pegRNA, whereas the RT template serves as a template for the synthesis of edited genetic information.8 epegRNA indicates the addition of engineered stabilizing structure at 3’-end of pegRNA for extended pegRNA expression and improved editing activity.10 (2) nCas9-RT. The SpCas9 nickase (nCas9) contains a H840A mutation to inactivate one of its two nuclease domains, thereby disabling its capability of making double-stranded DNA breaks and only allowing single strand DNA nicking. The Cas9 nickase is linked to a M-MLV RT capable of synthesizing DNA from a single-stranded RNA template. The nCas9-RT∗ in PEmax vectors designate optimization in codon usage, nuclear localization signals, and nCas9 activity through mutations. (3) sgRNA-nick is the sgRNA that directs the nCas9 to nick the nonedited DNA strand. The nick location of sgRNA-nick1 is 72 bp away from the pegRNA-induced nick. SgRNA-nick2 spacer partially overlaps with the pegRNA spacer and only matches with the PAM-containing strand after editing occurs, thereby minimizing indel frequency. (4) dominant negative MLH1 inhibits endogenous MLH1 through inhibition, thereby reducing cellular mismatch repair responses and increasing prime editing efficiency.9 Additional elements of the PE cassette include U6: U6 RNA polymerase III promoter; EF1α: Elongation factor 1α promoter, miRNA/β-3’UTR: miR-183-5p and miR-218-5p target sites embedded into β-globin to suppress nCas9-RT expression in HDAd producer cells, thus avoiding vector rearrangements, and supporting high vector production yields30; (pA1: BGH pA; pA2: SV40 pA, pA3: rabbit β-globin pA). The vectors also contain a PGK-MGMTP140K expression cassette used before.16 Note that PE3 is an earlier version of the prime editing system8 whereas PEmax consists of codon- and activity-optimized nCas9-RT components.9 (C) Analysis of G>A (silent PAM site) editing in cell lines. Human embryonic kidney (HEK) 293 and erythroid K562 cells (both without the SCD mutation) were transduced with HDAd vectors expressing either PE3, PE3max, PE3bmax, or PE5max at the indicated MOIs. Three days later, DNA was subjected to Sanger sequencing. Data shown here are from 2 independent experiments. (D) Editing of the target T>A site in Lin– BM cells from SCD CD46/Townes mice. Lin– cells, a fraction that is enriched for HSPCs, was infected with the 3 HDAd-PE vectors at an MOI of 500 vp/cell and editing was analyzed 4 days later using NGS of the target region. n = 3 donor mice; ∗P < .05. (E) Analysis of G>A (silent PAM site) editing in CD34+ cells from 3 healthy donors using NGS. Editing was measured at 4 days after transduction. MOI = 500 vp/cell. ∗P < .05. Statistical significance was assessed using one-way ANOVA with Šidák’s multiple comparisons test to calculate P values. pA, polyadenylation signals; UbC, human ubiquitin C promoter.31
HDAd vectors expressing prime editors and in vitro validation. (A) Intended base conversions. The goal is to convert the GTG (Val) codon to a GAA (Glu) codon to repair the sickle mutation (T>A) and to prevent the PE from continuing editing by destroying the PAM (G>A silent mutation). (B) Schematics of HDAd vectors used in this study. The prime editing machinery consists of (1) a prime editing guide RNA (pegRNA), capable of identifying the target nucleotide sequence to be edited and encoding new genetic information that replaces the targeted sequence. The pegRNA consists of an extended single guide RNA (sgRNA) containing a primer binding site (PBS) and a reverse transcriptase (RT) template sequence. During genome editing, the primer binding site allows the 3’-end of the nicked DNA strand to hybridize to the pegRNA, whereas the RT template serves as a template for the synthesis of edited genetic information.8 epegRNA indicates the addition of engineered stabilizing structure at 3’-end of pegRNA for extended pegRNA expression and improved editing activity.10 (2) nCas9-RT. The SpCas9 nickase (nCas9) contains a H840A mutation to inactivate one of its two nuclease domains, thereby disabling its capability of making double-stranded DNA breaks and only allowing single strand DNA nicking. The Cas9 nickase is linked to a M-MLV RT capable of synthesizing DNA from a single-stranded RNA template. The nCas9-RT∗ in PEmax vectors designate optimization in codon usage, nuclear localization signals, and nCas9 activity through mutations. (3) sgRNA-nick is the sgRNA that directs the nCas9 to nick the nonedited DNA strand. The nick location of sgRNA-nick1 is 72 bp away from the pegRNA-induced nick. SgRNA-nick2 spacer partially overlaps with the pegRNA spacer and only matches with the PAM-containing strand after editing occurs, thereby minimizing indel frequency. (4) dominant negative MLH1 inhibits endogenous MLH1 through inhibition, thereby reducing cellular mismatch repair responses and increasing prime editing efficiency.9 Additional elements of the PE cassette include U6: U6 RNA polymerase III promoter; EF1α: Elongation factor 1α promoter, miRNA/β-3’UTR: miR-183-5p and miR-218-5p target sites embedded into β-globin to suppress nCas9-RT expression in HDAd producer cells, thus avoiding vector rearrangements, and supporting high vector production yields30; (pA1: BGH pA; pA2: SV40 pA, pA3: rabbit β-globin pA). The vectors also contain a PGK-MGMTP140K expression cassette used before.16 Note that PE3 is an earlier version of the prime editing system8 whereas PEmax consists of codon- and activity-optimized nCas9-RT components.9 (C) Analysis of G>A (silent PAM site) editing in cell lines. Human embryonic kidney (HEK) 293 and erythroid K562 cells (both without the SCD mutation) were transduced with HDAd vectors expressing either PE3, PE3max, PE3bmax, or PE5max at the indicated MOIs. Three days later, DNA was subjected to Sanger sequencing. Data shown here are from 2 independent experiments. (D) Editing of the target T>A site in Lin– BM cells from SCD CD46/Townes mice. Lin– cells, a fraction that is enriched for HSPCs, was infected with the 3 HDAd-PE vectors at an MOI of 500 vp/cell and editing was analyzed 4 days later using NGS of the target region. n = 3 donor mice; ∗P < .05. (E) Analysis of G>A (silent PAM site) editing in CD34+ cells from 3 healthy donors using NGS. Editing was measured at 4 days after transduction. MOI = 500 vp/cell. ∗P < .05. Statistical significance was assessed using one-way ANOVA with Šidák’s multiple comparisons test to calculate P values. pA, polyadenylation signals; UbC, human ubiquitin C promoter.31
During HDAd amplification, high concentrations of transgene products were present in 116 producer cells particularly when strong promoters, such as the EF1α promoter, were used. In the case of genome editing enzymes, this appears to affect 116-cells viability resulting in the selection of rearranged HDAd vector genome with the editor expression cassette deleted or destroyed.14,30 To produce high-yield functional HDAd-PE vectors, PE expression in HDAd producer cells needed to be suppressed. This was achieved by placing the nCas9-RT gene under the regulation of miR-183-5p and miR-218-5p (which are expressed at high level in 116 cells but not in HSCs)30 and mi-vaRNAI (expressed from the helper virus in 116 cells).32 Corresponding microRNA binding sites were inserted into the 3’ untranslated region of the nCas6-RT gene so that the corresponding messenger RNA would be degraded in 116 cells but not in HSCs. In addition, we further modified the helper vector used for HDAd amplification in 116 cells by inserting expression cassettes for anti-CRISPR peptides capable of blocking Cas9 activity in part by preventing Cas9 binding to target DNA.33 The ability of such a combinatory approach to block PE activity in 116 cells is shown in supplemental Figure 2. Titers of HDAd-PE vectors were in the range of 0.5 × 1012 to 4.4 × 1012 viral particles (vp) (2.5 × 1012 vp on average) per 3-liter spinner flask culture. Expression of MLH-1 from HDAd-PE5max was confirmed through western blot analysis (supplemental Figure 3).
In vitro prime editing in cell lines and HSCs
The functionality of generated vectors was validated in cell lines. In human embryonic kidney (HEK293) cells and erythroid K562 cells with wild-type HBB allele, all vectors mediated the expected G>A base conversion at the silent PAM site 3 days after infection, with HDAd-PEmax vectors performing better than HDAd-PE3 (Figure 1C). At a multiplicity of infection (MOI) = 250 vp/cell, HDAd-PE3 generated 35% G>A conversion, whereas HDAd-PEmax vectors showed ∼100% conversion in K562 cells. The HDAd-PE3bmax vector using an alternative nicking strategy exhibited compromised editing activity compared with HDAd-PE3max. Therefore, it was excluded from downstream experiments. In lineage-negative cells (Lin– cells; a fraction enriched for HSCs) derived from CD46/Townes mice,23 HDAd-PE3, HDAd-PE3max, and HDAd-PE5max (MOI = 500 vp/cell) mediated 5.4%, 18.0%, and 25.1% correction of the sickle cell mutation at day 4 after infection, respectively (Figure 1D). This trend of editing activity (HDAd-PE3<HDAd-PE3max<HDAd-PE5max) was also observed in human CD34+ HSPCs from mobilized healthy donors, however with an overall lower editing rate (G>A) at the silent PAM site), which was 1.0%, 1.6%, and 3.4%, respectively (Figure 1D). Based on the in vitro data, the HDAd-PE5max vector was chosen for further studies.
In vitro prime editing in CD34+ cells from patients with SCD
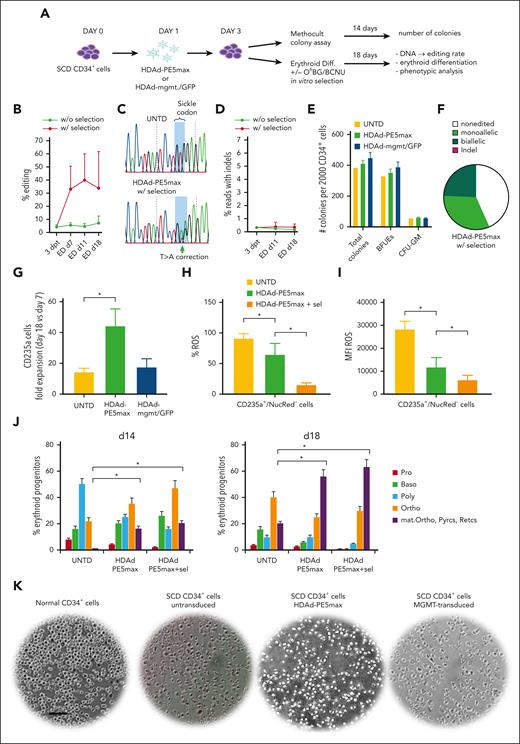
CD34+ cells from 3 patients with SCD (β0/βS, βS/βS, and βS/βS) were isolated from exchange transfusion bags. After transduction with HDAd-PE5max, cells were subjected to in vitro ED with or without in vitro O6BG/BCNU selection (Figure 2A). Without selection, an average of 4.6% and 7.5% of T>A conversion was detected in βS/βS samples at 3 days after transduction and 18 days of ED, respectively; editing rate (GTG>GAA) in the β0/βS donor was at a similar level. Selection through 1 round treatment with O6BG/BCNU was performed in cells derived from βS/βS donors. After selection, the conversion rate reached an average of 33.9% at the end of ED (Figure 2B-C). The frequency of indels was <0.5% at day 3 after transduction and did not increase during ED (Figure 2D). HDAd-PE5max transduction had no significant effect on progenitor colony formation when compared with untransduced cells and HDAd-mgmt/green fluorescent protein (GFP) control vector–transduced cells (Figure 2E). With HDAd-PE5max transduction and selection, 24.6% and 32.4% colonies derived from donor 3 showed bi- and monoallelic correction of the sickle mutation, respectively (Figure 2F). From day 7 to day 18 of ED the number of erythroid CD235a+ cells increased on average ∼45-fold for HDAd-PE5max transduced CD34+ cells, whereas untransduced or control vector transduced cells expanded only 18- to 19-fold (Figure 2G; supplemental Figure 4A). At the end of ED, CD235a+/NucRed– cells derived from HDAd-PE5max transduced CD34+ cells showed significantly lower levels of ROS, a hallmark of oxidative stress as seen in SCD34 (Figure 2H-I; supplemental Figure 4B). HDAd-PE5max edited cells also displayed improved ED as supported by microscopic analyses of cells during days 14 and 18 of ED, which showed more mature and differentiated cells (orthochromatic erythroblasts, reticulocytes, and pyrenocytes) (Figure 2J; supplemental Figure 5) and reduced sickling after sodium metabisulfite challenge at the end of ED culture (Figure 2K).
In vitro studies with CD34+ cells from patients with SCD infected with HDAd-PE5max. (A) SCD CD34+ cells (from 3 donors) were infected with HDAd-PE5max or the control virus HDAd-mgmt/GFP at an MOI of 4000 vp/cell or left untransduced (UNTD). On day 3, cells were either plated in Methocult for progenitor colony assays or subjected to ED with or without O6BG/BCNU in vitro selection. (B) Editing rates measured using NGS at various time points after transduction and in vitro differentiation with or without selection. (C) DNA chromatograms from Sanger sequencing showing target site T>A conversion in cells of donor #3 after ED. (D) Percentage of reads with indels. (E) Number of progenitor colonies formed from 2000 plated CD34+ cells (counted at day 14 of culture). BFUE, blast-forming units-erythroid; CFU-GM, granulocyte-macrophage colony–forming unit. (F) Editing measured using Sanger sequencing in donor #3 progenitor colonies after HDAd-PE5max transduction and selection (n = 111). (G) Fold expansion of total cell at day 18 of ED vs day 7 of ED. N = 3 donors. (H and I) Flow cytometry for ROS at day 18 of ED of untransduced cells and cells transduced with HDAd-PE5max without and with O6BG/BCNU in vitro selection (sel). (H) Percentage of ROS-positive cells. (I) ROS mean fluorescence intensity. (J) Percentage of erythroid progenitors counted on cytospins from differentiated SCD CD34+ cells at days 14 and 18 of ED, untransduced (UNTD) and HDAd-PE5max–transduced without and with selection. Two scientists counted 5 random fields. (K) Smears of total blood cells subjected to a sodium metabisulfite sickling assay. Pictures shown here are from day 18 of ED. The scale bar is 25 μm. ∗P < .05. Statistical significance was assessed using one-way ANOVA with Šidák’s multiple comparisons test to calculate P values. Baso, basophilic erythroblasts; dpt, days post transduction; Diff, in vitro ED; mat. Ortho, maturing orthochromatic erythroblasts; Ortho, orthochromatic erythroblasts; Poly, polychromatic erythroblasts; Pro, proerythroblasts; Pyrcs, pyrenocytes; Retics, reticulocytes.
In vitro studies with CD34+ cells from patients with SCD infected with HDAd-PE5max. (A) SCD CD34+ cells (from 3 donors) were infected with HDAd-PE5max or the control virus HDAd-mgmt/GFP at an MOI of 4000 vp/cell or left untransduced (UNTD). On day 3, cells were either plated in Methocult for progenitor colony assays or subjected to ED with or without O6BG/BCNU in vitro selection. (B) Editing rates measured using NGS at various time points after transduction and in vitro differentiation with or without selection. (C) DNA chromatograms from Sanger sequencing showing target site T>A conversion in cells of donor #3 after ED. (D) Percentage of reads with indels. (E) Number of progenitor colonies formed from 2000 plated CD34+ cells (counted at day 14 of culture). BFUE, blast-forming units-erythroid; CFU-GM, granulocyte-macrophage colony–forming unit. (F) Editing measured using Sanger sequencing in donor #3 progenitor colonies after HDAd-PE5max transduction and selection (n = 111). (G) Fold expansion of total cell at day 18 of ED vs day 7 of ED. N = 3 donors. (H and I) Flow cytometry for ROS at day 18 of ED of untransduced cells and cells transduced with HDAd-PE5max without and with O6BG/BCNU in vitro selection (sel). (H) Percentage of ROS-positive cells. (I) ROS mean fluorescence intensity. (J) Percentage of erythroid progenitors counted on cytospins from differentiated SCD CD34+ cells at days 14 and 18 of ED, untransduced (UNTD) and HDAd-PE5max–transduced without and with selection. Two scientists counted 5 random fields. (K) Smears of total blood cells subjected to a sodium metabisulfite sickling assay. Pictures shown here are from day 18 of ED. The scale bar is 25 μm. ∗P < .05. Statistical significance was assessed using one-way ANOVA with Šidák’s multiple comparisons test to calculate P values. Baso, basophilic erythroblasts; dpt, days post transduction; Diff, in vitro ED; mat. Ortho, maturing orthochromatic erythroblasts; Ortho, orthochromatic erythroblasts; Poly, polychromatic erythroblasts; Pro, proerythroblasts; Pyrcs, pyrenocytes; Retics, reticulocytes.
Correction of the SCD mutation by ex vivo HSC transduction. (A) Schematic of the experiment. BM Lin− cells were harvested from CD46/Townes mice and transduced with HDAd-PE5max at an MOI of 500 vp/cell. Cells were then either cultured for 3 days or transplanted into lethally irradiated C57BL/6 mice at 24 hours after transduction. The mice were followed for 16 weeks. For further evaluation of long-term repopulating cells, BM Lin− cells from these mice were then used for secondary transplantation and these mice were monitored for another 16 weeks. (B and C) Analyses of day 3 cultures and colonies. (B) Target base conversions and indel frequency measured using NGS. Lin− cells isolated from 3 independent donors were analyzed (n = 3 animals). (C) Allelic analysis in single Lin– cell–derived progenitor colonies (n = 30). Editing was measured using Sanger sequencing at day 11 after plating of transduced Lin– cells derived from a representative mouse. (D-H) Analyses of transplanted primary mice. (D) Engraftment of HDAd-PE5max–transduced HSCs measured using flow cytometry of human CD46 expression in PBMCs. (E) Analysis of target site editing (T>A, sickle site and G>A, silent PAM site) using Sanger sequencing in PBMCs at different time points after transplantation (n = 5 animals). (F) Editing (Sanger) at week 16 in PBMCs, splenocytes, BM MNCs, BM Lin– cells and pooled progenitor colonies from plated Lin– cells (Pooled CFU). (G) Target base conversions and indel frequency in BM MNCs (week 16) measured using NGS. (H) Allelic analysis in progenitor colonies derived from week-16 BM Lin– cells (n = 36). Colonies from 3 individual mice were analyzed using Sanger sequencing (n = 12 for each mouse). All colonies had a 100% editing rate. (I-K). Analyses of secondary recipients similar to panels D-H. For panels C, E, F, H, J, and K, editing was measured using Sanger sequencing. For panels D-G, I-K, each symbol represents an individual mouse.
Correction of the SCD mutation by ex vivo HSC transduction. (A) Schematic of the experiment. BM Lin− cells were harvested from CD46/Townes mice and transduced with HDAd-PE5max at an MOI of 500 vp/cell. Cells were then either cultured for 3 days or transplanted into lethally irradiated C57BL/6 mice at 24 hours after transduction. The mice were followed for 16 weeks. For further evaluation of long-term repopulating cells, BM Lin− cells from these mice were then used for secondary transplantation and these mice were monitored for another 16 weeks. (B and C) Analyses of day 3 cultures and colonies. (B) Target base conversions and indel frequency measured using NGS. Lin− cells isolated from 3 independent donors were analyzed (n = 3 animals). (C) Allelic analysis in single Lin– cell–derived progenitor colonies (n = 30). Editing was measured using Sanger sequencing at day 11 after plating of transduced Lin– cells derived from a representative mouse. (D-H) Analyses of transplanted primary mice. (D) Engraftment of HDAd-PE5max–transduced HSCs measured using flow cytometry of human CD46 expression in PBMCs. (E) Analysis of target site editing (T>A, sickle site and G>A, silent PAM site) using Sanger sequencing in PBMCs at different time points after transplantation (n = 5 animals). (F) Editing (Sanger) at week 16 in PBMCs, splenocytes, BM MNCs, BM Lin– cells and pooled progenitor colonies from plated Lin– cells (Pooled CFU). (G) Target base conversions and indel frequency in BM MNCs (week 16) measured using NGS. (H) Allelic analysis in progenitor colonies derived from week-16 BM Lin– cells (n = 36). Colonies from 3 individual mice were analyzed using Sanger sequencing (n = 12 for each mouse). All colonies had a 100% editing rate. (I-K). Analyses of secondary recipients similar to panels D-H. For panels C, E, F, H, J, and K, editing was measured using Sanger sequencing. For panels D-G, I-K, each symbol represents an individual mouse.
Therapeutic prime editing in CD46/Townes mice after in vivo HSC transduction with HDAd-PE5max. (A) Experimental procedure. Mice (n = 7) were mobilized using G-CSF/AMD3100 and were transduced in vivo with HDAd-PE5max (8 × 1010 vp/mouse = 3.2 × 1012 vp/kg). For in vivo selection, mice were injected with O6-BG (15 mg/kg, intraperitoneal [IP]) 2 times, 30 minutes apart. One hour after the second injection of O6-BG, mice were injected with BCNU (5 mg/kg, IP). For the second and third cycle, BCNU concentrations were increased to 9 mg/kg and 10 mg/kg, respectively. The mice were killed during week 16 for analyses. Lin– cells were isolated from BM and IV injected into lethally irradiated C57BL/6J mice. The secondary transplanted mice were followed for another 16 weeks for the specified terminal point analyses. (B-F) Editing measured using Sanger sequencing of in vivo–transduced mice. (B) Target base editing (sickle repair and silent PAM) in PBMCs. (C) Editing in cells from different tissues at week 16 after in vivo transduction. (D) Editing in different lineage-positive cell subsets and Lin– cells at week 16. CD3+ T cells, CD19+ B cells, Gr1+ granulocytes, Ter-119+ erythroid cells were sorted from BM MCs using flow cytometry. (E) Allelic analysis in single Lin– cell–derived progenitor colonies. Editing was measured at day 11 after plating of transduced Lin– cells. Data from 4 mice with indicated ear tag number are shown. Twelve colonies for each mouse were analyzed. (F) Editing in tissues (n = 4). (G-I) Analyses of secondary recipients. Lin– cells from in vivo–transduced mice were transplanted into lethally irradiated C57BL/6 mice (n = 7) (cells from donor into 1 recipient). (G) Engraftment based on human CD46 expression in PBMCs. (H) Editing measured using Sanger sequencing in PBMCs of secondary recipients at indicated weeks after transplantation. (I) Editing measured using Sanger sequencing at week 16 after transplantation in PBMCs, splenocytes, and BM cells. (J) Target base conversions and indel frequency measured using NGS. Week 16 BM MNCs samples from primary and secondary mice were used. K-N) Analyses of hemoglobin composition. Whole blood samples at week 16 after in vivo (n = 7) and ex vivo (n = 5) transduction were analyzed. Samples from untreated CD46/Townes mice (n = 3) were used as control. (K) Percentages of hemoglobin tetramers as measured using HPLC. Representative chromatograms are shown in supplemental Figure 12A. (L) Percentages of hemoglobin subunits as measured using mass spectrometry. (M) Representative chromatogram pattern showing the separation of βA from βS globin chains. The labeled percentages were calculated based on the peak areas. See supplemental Figure 12B for representative full spectrum chromatograms. (N) Separation of hemoglobin variants using isoelectric focusing electrophoresis. Each lane represents 1 mouse (ear tag number labeled) or AFSC controls. Bands in AFSC controls indicating 4 different hemoglobin variants are labeled. For B-D and F-J, each dot represents 1 animal. Data shown are mean ± standard deviation, wherever applicable. AFSC, hemoglobin A, F, S, and C controls.
Therapeutic prime editing in CD46/Townes mice after in vivo HSC transduction with HDAd-PE5max. (A) Experimental procedure. Mice (n = 7) were mobilized using G-CSF/AMD3100 and were transduced in vivo with HDAd-PE5max (8 × 1010 vp/mouse = 3.2 × 1012 vp/kg). For in vivo selection, mice were injected with O6-BG (15 mg/kg, intraperitoneal [IP]) 2 times, 30 minutes apart. One hour after the second injection of O6-BG, mice were injected with BCNU (5 mg/kg, IP). For the second and third cycle, BCNU concentrations were increased to 9 mg/kg and 10 mg/kg, respectively. The mice were killed during week 16 for analyses. Lin– cells were isolated from BM and IV injected into lethally irradiated C57BL/6J mice. The secondary transplanted mice were followed for another 16 weeks for the specified terminal point analyses. (B-F) Editing measured using Sanger sequencing of in vivo–transduced mice. (B) Target base editing (sickle repair and silent PAM) in PBMCs. (C) Editing in cells from different tissues at week 16 after in vivo transduction. (D) Editing in different lineage-positive cell subsets and Lin– cells at week 16. CD3+ T cells, CD19+ B cells, Gr1+ granulocytes, Ter-119+ erythroid cells were sorted from BM MCs using flow cytometry. (E) Allelic analysis in single Lin– cell–derived progenitor colonies. Editing was measured at day 11 after plating of transduced Lin– cells. Data from 4 mice with indicated ear tag number are shown. Twelve colonies for each mouse were analyzed. (F) Editing in tissues (n = 4). (G-I) Analyses of secondary recipients. Lin– cells from in vivo–transduced mice were transplanted into lethally irradiated C57BL/6 mice (n = 7) (cells from donor into 1 recipient). (G) Engraftment based on human CD46 expression in PBMCs. (H) Editing measured using Sanger sequencing in PBMCs of secondary recipients at indicated weeks after transplantation. (I) Editing measured using Sanger sequencing at week 16 after transplantation in PBMCs, splenocytes, and BM cells. (J) Target base conversions and indel frequency measured using NGS. Week 16 BM MNCs samples from primary and secondary mice were used. K-N) Analyses of hemoglobin composition. Whole blood samples at week 16 after in vivo (n = 7) and ex vivo (n = 5) transduction were analyzed. Samples from untreated CD46/Townes mice (n = 3) were used as control. (K) Percentages of hemoglobin tetramers as measured using HPLC. Representative chromatograms are shown in supplemental Figure 12A. (L) Percentages of hemoglobin subunits as measured using mass spectrometry. (M) Representative chromatogram pattern showing the separation of βA from βS globin chains. The labeled percentages were calculated based on the peak areas. See supplemental Figure 12B for representative full spectrum chromatograms. (N) Separation of hemoglobin variants using isoelectric focusing electrophoresis. Each lane represents 1 mouse (ear tag number labeled) or AFSC controls. Bands in AFSC controls indicating 4 different hemoglobin variants are labeled. For B-D and F-J, each dot represents 1 animal. Data shown are mean ± standard deviation, wherever applicable. AFSC, hemoglobin A, F, S, and C controls.
In summary, transduction of CD34+ cells from patients with SCD with HDAd-PE5max mediates efficient correction of the SCD mutation resulting in phenotypic amelioration of sickle cell–derived erythropoiesis.
Highly efficient ex vivo HSC prime editing in CD46/Townes mice without selection
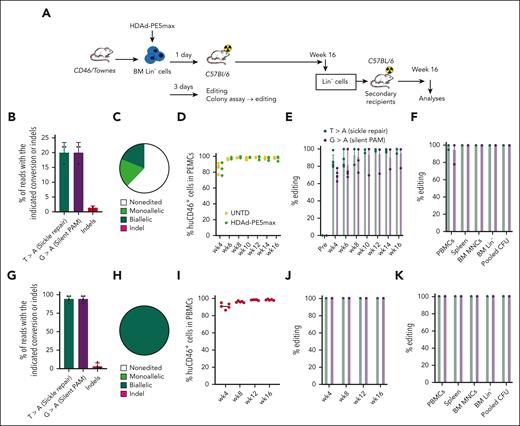
Next, we tested an ex vivo prime editing approach. BM Lin− cells from CD46/Townes mice were transduced with HDAd-PE5max (MOI = 500 vp/cell). A fraction of the cells was cultured in vitro for measuring editing in the transplant and MethoCult colony assay (Figure 3A). At day 3 after transduction, 20.2% T>A correction at the sickle mutation site was detected (Figure 3B). The frequency of indels at the target site was 1.5% on average, which was 13.5-fold lower than the prime editing frequency (Figure 3B). In single Lin– cell–derived progenitor colonies (n = 30), 37.6% of clones showed at least one edited allele; half of the edited clones contained biallelic editing and no colonies showed indels (Figure 3C). The remaining cells were infused into lethally irradiated C57BL/6 mice which were followed for 16 weeks (Figure 3A). Engraftment, measured based on human CD46 expression on PBMCs, was >95% after week 6 and was similar to untransduced Lin– cells in control mice, indicating that ex vivo HSC transduction and editing did not affect HSC functions (Figure 3D). After transplantation, T>A frequencies measured in PBMCs by Sanger sequencing were >85% at the first time point analyzed (4 weeks after transplantation) and remained stable over the 16-week monitoring period (Figure 3E). Notably, this was achieved without O6BG/BCNU selection. At week 16, ∼100% editing frequencies were detected in splenocytes, total BM mononuclear cells (BM MNCs), BM Lin– cells, and Lin– cell–derived colonies (Figure 3F). BM MNCs were subjected to NGS; 94.6% of T>A and 94.4% of G>A conversion rates were found at the sickle mutation site and the silent PAM site, respectively. The frequency of indels was on an average of 3.5% (0.2% to 9.9%), 26.8-fold lower than the desired edit (Figure 3G). In Lin– cell–derived colonies during week 16, biallelic prime editing was detected in all analyzed colonies (Figure 3H). To study long-term effects of ex vivo transduction, Lin– cells harvested from week-16 primary mice were subjected to transplantation into secondary recipients which were followed for another 16 weeks (Figure 3A). Near complete engraftment (Figure 3I) and stable prime edits (Figures 3J-K) were maintained for the 16-week observation period, suggesting that gene editing occurred in long-term repopulating stem cells. Furthermore, no dramatic differences in BM lineage composition and percentage of HSCs (lineage [Lin]− Sca-1+ c-Kit+ [LSK] cells) were found between untreated and treated animals (supplemental Figure 6). Analyses of correction at the protein level and systematic phenotypic evaluation is described in the following section that covers data from both the ex vivo and in vivo approaches.
Taken together, HDAd-PE5max confers efficient ex vivo HSC editing with near complete correction of the sickle cell mutation in the transplanted mice without any O6BG/BCNU selection.
Therapeutic prime editing after in vivo HSC transduction and selection
We next sought to directly fix the sickle mutation in vivo in CD46/Townes mice by IV delivering the nonintegrating HDAd-PE5max vector after HSC mobilization with G-CSF/AMD3100. We used a new in vivo HSC transduction or selection strategy with O6BG/BCNU treatment being started at day 6 and repeated at days 19 and 33, capitalizing on MGMTP140K expression from episomal HDAd-PE5max genomes (Figure 4A). The basis for this regimen was an in vivo HSC transduction kinetics study with an HDAd-mgmt/GFP vector (supplemental Figure 7) showing that the percentage of GFP-positive BM LSK cells declined only 30% to 50% from day 6 to day 33. Similar kinetics were also detectable at the DNA (vector copy number [VCN]) and messenger RNA levels. In vivo selection did not significantly affect the transgene expression kinetic from episomal vectors.
Prime editing in PBMCs was measured using Sanger sequencing. The T>A conversion rate at the sickle mutation site reached to an average of 30.9% at week 6 and 43.6% at week 16 after in vivo HSC transduction or selection (Figure 4B). At week 16, this therapeutically relevant level of editing was consistently detected in splenocytes, BM MNCs, BM Lin– cells, and Lin– cell–derived colonies (Figure 4C), as well as in lineage-positive cell subsets, including CD3+ T cells, CD19+ B cells, Gr-1+ myeloid cells, and Ter-119+ erythroid cells (Figure 4D), demonstrating that prime editing occurred in multipotent HSCs. We analyzed editing in colonies derived from single BM Lin– cells of 4 individual mice. An average of 31.1% (20% to 50%) and 33.1% (8.3% to 58.3%) colonies (n = 12 for each animal) showed biallelic and monoallelic T>A correction, respectively (Figure 4E). No colony (n = 48) was found to contain indels. β-Globin target site editing analyzed in lysates of colon, kidney, heart, and brain cells was detected by Sanger sequencing in some animals (Figure 4F). Average editing rates in liver and lung cells were ∼20% and 25%. These observations are not surprising considering that these tissues contain high numbers of circulating blood cells and that transduced or edited HSCs differentiate into residential tissue macrophages.35 No editing was detected in the cells derived from ovaries. Future in vivo prime editing studies will be conducted in male Townes/CD46 mice to exclude editing in the cells derived from testis. Notably, previous in vivo HSC transduction or selection studies in 4 rhesus macaques with integrating vectors expressing γ-globin did not detect vector DNA in the cells derived from testis.36
To study the long-term effect of prime editing, BM Lin– cells isolated from in vivo–transduced “primary” mice at week 16 were transplanted into lethally irradiated C57BL/6 recipients and monitored for another 16 weeks. Engraftment was >95% after week 6 (Figure 4G). The genetic correction frequency in PBMCs was stably maintained at ∼40% on an average during the 16-week observation period (Figure 4H), and a consistent level of correction was detected in different tissues and BM Lin− cells at week 16 after engraftment (Figure 4I), demonstrating that PE-mediated correction occurred in long-term repopulating HSCs. To examine the product purity of in vivo prime editing, the target region, amplified using week-16 BM MNCs, was subjected to amplicon NGS. We found that nearly all edited alleles contained the desired GTG>GAA conversion (supplemental Figure 8A). Indels occurred around the predicted nicking sites and their frequencies were averaged at 1.4% and 1.6% in primary and secondary mice, respectively, >20-fold lower than prime editing levels (Figure 4J; supplemental Figure 8B). Compared with a previous study with integrated MGMTP140K expression,23 vector copy number in BM MNCs was 0.005 copies/cell or 620-fold lower in in vivo–transduced primary mice, and a further 5.7-fold drop was measured in secondary recipients (supplemental Figure 9A). No vector integration was detected by inverse polymerase chain reaction in BM MNCs of secondary recipients at week 16 (supplemental Figure 9B). The origin of the measured (close-to background) vector genome signals needs further investigation. In vivo HSC prime editing did not cause obvious changes in activity, body weight, and clinical presentations. In addition, no significant differences in BM lineage composition and percentage of HSCs (LSK cells) were found between untreated and treated animals (supplemental Figure 10). Furthermore, we performed in vivo HSC transduction with the HDAd-PE3max vector and found that, despite at a lower editing frequency, HDAd-PE3max mediated efficient in vivo HSC prime editing (∼18%) without the involvement of the dominant-negative MLH1 gene (supplemental Figure 11). These data collectively demonstrate that the sickle mutation was corrected in vivo in HSCs with HDAd-PE5max at a level of therapeutic relevance.
Correction at the protein level
Because both α- and β-globin in CD46/Townes mice were replaced with human orthologs, we measured hemoglobin tetramers in mouse blood samples using ion-exchange high-performance liquid chromatography (HPLC). In untreated CD46/Townes blood samples, the predominant form of hemoglobin was HbS (>98%). After ex vivo treatment with HDAd-PE5max, >99% of hemoglobin tetramers transformed to HbA, in agreement with genomic correction rates. More importantly, for blood samples collected from mice transduced in vivo with HDAd-PE5max (week 16), an average of 43% correction (HbS > HbA) was detected (Figure 4K; supplemental Figure 12A). This is above the required threshold for a cure.37 To differentiate the corrected βA from the sickle βS subunit and quantitate their ratio, we developed another method using mass spectrometry based on their different mass-to-charge ratios and observed similar levels of correction at the globin subunit level (Figure 4L-M; supplemental Figure 12B). We further verified the composition of hemoglobin tetramers using isoelectric focusing (Figure 4N). These data confirm near complete correction of the HbS at hemoglobin protein level in the ex vivo setting. After in vivo prime editing with HDAd-PE5max and selection, >40% therapeutic correction was achieved.
Phenotypic correction in the SCD mouse model after ex vivo and in vivo prime editing
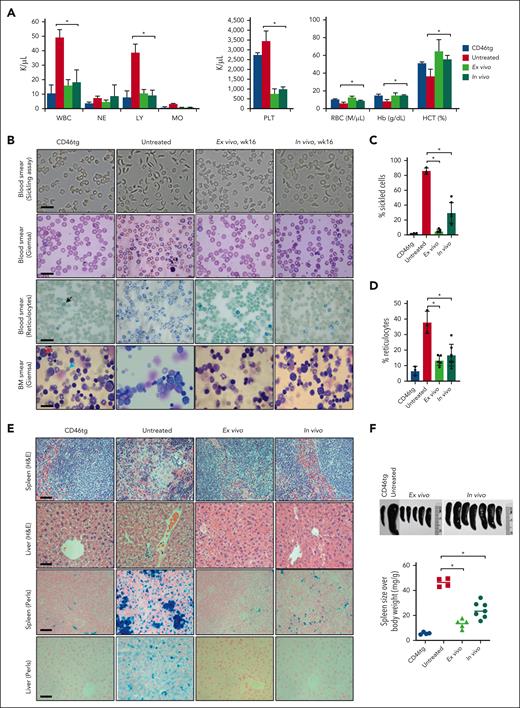
We evaluated phenotypic changes before and after prime editing with HDAd-PE5max. Whole blood samples from naïve CD46tg mice (n = 3; as a healthy control), untreated CD46/Townes mice (n = 3), ex vivo treated mice (n = 5; week 16; primary recipients), and in vivo–transduced mice (n = 7; week 16) were analyzed for hematological parameters. Untreated mice showed high leukocytosis, low RBC count, and low hematocrit and hemoglobin production in comparison with CD46tg healthy mice. Importantly, CD46/Townes mice edited using HDAd-PE5max through both ex vivo and in vivo strategies, exhibited normal hematological parameters at levels comparable with that of the healthy controls (Figure 5A).
Phenotypic correction of CD46/Townes mice using ex vivo and in vivo approaches at week 16 after treatment. (A) Blood cell analysis of healthy control CD46tg mice (“CD46tg,” n = 3), untreated CD46/Townes mice (“untreated,” n = 3), primary recipient mice at week 16 after transplantation of ex vivo–transduced BM Lin− cells from CD46/Townes mice (“ex vivo,” n = 5), and CD46/Townes mice at week 16 after in vivo transduction (“in vivo,” n = 7). (B) Representative microphotographs of blood cell and BM smears. First panel: smears of total blood cells subjected to a sickling assay; second panel: blood cell smears stained with Giemsa; third panel: staining of blood smears for reticulocytes with brilliant cresyl blue, which stains nuclear remnants of basophilic ribonucleoproteins in reticulocytes (black arrow); fourth panel: BM smears stained with Giemsa. The red arrow marks an undifferentiated proerythroblast. The blue arrow points at more differentiated erythroblasts or reticulocytes. The scale bars are 20 μm. (C) Percentage of sickle cells in blood smears. Each dot represents the percentage in an individual mouse. (D) Percentage of reticulocytes in blood smears. (E) Upper 2 panels: spleen and liver sections stained with hematoxylin and eosin. Lower 2 panels: tissue hemosiderosis visualized using Perls Prussian blue staining. Iron deposition is shown as cytoplasmic blue pigments of hemosiderin in spleen tissue sections. The scale bars are 200 μm. (F) Spleen sizes (upper panel) and spleen weight relative to body weight (lower panel). Each symbol represents an individual mouse. ∗P < .05. Statistical differences were calculated using one-way ANOVA with Šidák’s multiple comparisons test. HCT, hematocrit; Hb, hemoglobin; LY, lymphocytes; MO, monocytes; NE, neutrophils; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
Phenotypic correction of CD46/Townes mice using ex vivo and in vivo approaches at week 16 after treatment. (A) Blood cell analysis of healthy control CD46tg mice (“CD46tg,” n = 3), untreated CD46/Townes mice (“untreated,” n = 3), primary recipient mice at week 16 after transplantation of ex vivo–transduced BM Lin− cells from CD46/Townes mice (“ex vivo,” n = 5), and CD46/Townes mice at week 16 after in vivo transduction (“in vivo,” n = 7). (B) Representative microphotographs of blood cell and BM smears. First panel: smears of total blood cells subjected to a sickling assay; second panel: blood cell smears stained with Giemsa; third panel: staining of blood smears for reticulocytes with brilliant cresyl blue, which stains nuclear remnants of basophilic ribonucleoproteins in reticulocytes (black arrow); fourth panel: BM smears stained with Giemsa. The red arrow marks an undifferentiated proerythroblast. The blue arrow points at more differentiated erythroblasts or reticulocytes. The scale bars are 20 μm. (C) Percentage of sickle cells in blood smears. Each dot represents the percentage in an individual mouse. (D) Percentage of reticulocytes in blood smears. (E) Upper 2 panels: spleen and liver sections stained with hematoxylin and eosin. Lower 2 panels: tissue hemosiderosis visualized using Perls Prussian blue staining. Iron deposition is shown as cytoplasmic blue pigments of hemosiderin in spleen tissue sections. The scale bars are 200 μm. (F) Spleen sizes (upper panel) and spleen weight relative to body weight (lower panel). Each symbol represents an individual mouse. ∗P < .05. Statistical differences were calculated using one-way ANOVA with Šidák’s multiple comparisons test. HCT, hematocrit; Hb, hemoglobin; LY, lymphocytes; MO, monocytes; NE, neutrophils; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
To examine the RBC morphology, the above blood samples were subjected to an in vitro sickling assay with sodium metabisulphite, a reagent that reduces oxygen tension, consequently inducing the typical sickle-shape of RBCs derived from patients with SCD or derived from SCD mice. More than 86% of RBCs from untreated CD46/Townes mice showed an elongated, crescent, or sickled shape (Figure 5B, top panel). Compared with the healthy control, ex vivo–treated mouse samples showed no significant sickling. The percentage of sickled cells in in vivo–transduced blood samples dropped to 29.6%, significantly lower than that of untreated mouse samples (Figure 5B-C). Accordingly, ex vivo and in vivo transduction with HDAd-PE5max markedly reduced the percentages of reticulocytes in blood smears from 37.7% to 13.3% and 16.7%, respectively (Figure 5B-D). Similarly, BM cytospins stained with Giemsa showed more matured erythroblasts or reticulocytes seen in healthy CD46tg and in prime edited samples (Figure 5B, fourth panel).
Histological analyses of liver and spleen tissues from prime edited CD46/Townes mice showed normal architecture with largely regressed parenchymal iron deposition and extramedullary hemopoiesis (Figure 5E), in sharp contrast to untreated CD46/Townes tissue sections. Splenomegaly, resulting from compensatory hemopoiesis, is a characteristic disease symptom seen in patients with SCD and in CD46/Townes mice.23 Compared with untreated CD46/Townes mice, a marked reduction of spleen size was seen in prime edited animals (Figure 5F). Taken together, direct repair of the sickle mutation through ex vivo and in vivo prime editing with HDAd-PE5max in HSCs significantly ameliorated the SCD phenotype in CD46/Townes mice.
OT editing analyses
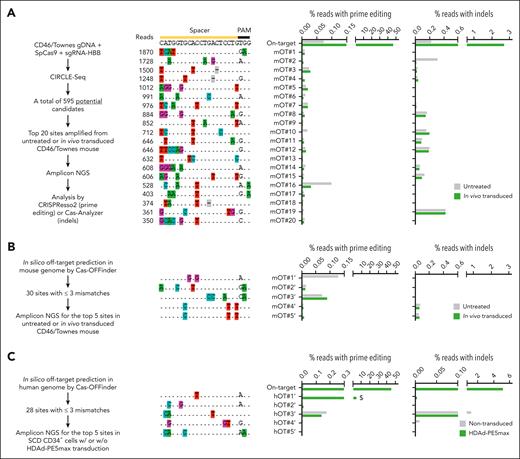
First, we examined the fidelity of HDAd-PE5max using CIRCLE-seq, a highly sensitive in vitro method for genome-wide OT screening.38 Fragmented and then circularized genomic DNA of CD46/Townes mice was cleaved using the wild-type SpCas9 coupled with HBBS-targeting sgRNA which was contained in the HDAd-PE5max vector. The cleavage led to specific linearization of targetable regions which were subsequently barcoded and deep sequenced. CIRCLE-seq identified a total of 595 potential OT sites, one of which had 2-bp mismatches and the rest had 3 to 6-bp mismatches in the spacer region (Figure 6A, left workflow; supplemental Table 2). Next, we performed targeted amplicon sequencing of the top 20 sites of the 595 candidates in in vivo–transduced animals. Compared with an untreated mouse, the in vivo–transduced mouse with the highest on-target editing (46.5% T>A correction) did not show markedly high nucleotide alterations associated with prime editing at these top OT sites (Figure 6A, middle panel). Notably, indel frequencies around the nicking sites were generally <0.2% and comparable with control samples (Figure 6A, right panel). Second, we analyzed OT editing using Cas-OFFinder, a computational algorithm that can search for potential OT sites of the sickle mutation–targeting sgRNA. A total of 30 sites with mismatches ≤3 bp were nominated in the mouse genome (supplemental Table 3). Of this list, 19 sites overlapped with candidates identified using CIRCLE-seq. The top 5 Cas-OFFinder sites were experimentally examined using amplicon deep sequencing and revealed no detectable prime editing or indel formation in in vivo–transduced samples (Figure 6B). Third, we examined top-scored OT sites in SCD CD34+ cells. Cas-OFFinder predicted a list of 28 sites with mismatches ≤3 bp in the human genome (supplemental Table 4). Targeted amplicon sequencing of the top 5 human sites uncovered that only the top-ranked site (hOT#1’) showed substantial prime editing (4.4%; >10-fold lower than the 45.5% on-target editing) in SCD CD34+ cells after HDAd-PE5max transduction (Figure 6C). This site maps to the HBD gene and has a 1 bp mismatch with the on-target HBB sequence. Nevertheless, the prime editing base conversion at this site (GAG>GAA at the 6 codon) does not change the HBD amino acid sequence. These observations together reveal no concerning OT editing after HDAd-PE5max treatment in vivo in CD46/Townes mice and in vitro in SCD CD34+ cells.
Analyses of OT effects. (A) OT editing measured using amplicon deep sequencing at the top 20 potential CIRCLE-seq nominated sites. The workflow is illustrated on the left side. Genomic DNA was isolated from BM MNCs of an untreated mouse or the mouse showing highest on-target editing after in vivo transduction with HDAd-PE5max. OT prime editing (middle bar graph) was calculated based on the percentage of reads with the G/T/C > A conversion at position +4 (corresponding to sickle mutation), counting the predicted nicking site as position +1. If position +4 was already an A in the wild-type allele, the calculation was performed based on the percentage of G/T/C > A conversion at position +5 (corresponding to the silent PAM mutation). Percentage of reads with indels was also analyzed (right bar graph). (B) OT editing at the top-5 ranked potential sites in mouse genome nominated through in silico prediction using Cas-OFFinder. Genomic DNA samples were the same as those in A. The workflow is illustrated on the left side. (C) OT editing at the top-5 ranked potential sites in human genome nominated through in silico prediction using Cas-OFFinder. Genomic DNA was isolated from untreated or HDAd-PEmax–transduced and selected SCD CD34+ cells. Percentage of reads with prime editing and indels are shown. Note that hOT#1’ (highlighted by a $ symbol) maps to the HBD site and only bears 1 bp mismatch at the on-target site.
Analyses of OT effects. (A) OT editing measured using amplicon deep sequencing at the top 20 potential CIRCLE-seq nominated sites. The workflow is illustrated on the left side. Genomic DNA was isolated from BM MNCs of an untreated mouse or the mouse showing highest on-target editing after in vivo transduction with HDAd-PE5max. OT prime editing (middle bar graph) was calculated based on the percentage of reads with the G/T/C > A conversion at position +4 (corresponding to sickle mutation), counting the predicted nicking site as position +1. If position +4 was already an A in the wild-type allele, the calculation was performed based on the percentage of G/T/C > A conversion at position +5 (corresponding to the silent PAM mutation). Percentage of reads with indels was also analyzed (right bar graph). (B) OT editing at the top-5 ranked potential sites in mouse genome nominated through in silico prediction using Cas-OFFinder. Genomic DNA samples were the same as those in A. The workflow is illustrated on the left side. (C) OT editing at the top-5 ranked potential sites in human genome nominated through in silico prediction using Cas-OFFinder. Genomic DNA was isolated from untreated or HDAd-PEmax–transduced and selected SCD CD34+ cells. Percentage of reads with prime editing and indels are shown. Note that hOT#1’ (highlighted by a $ symbol) maps to the HBD site and only bears 1 bp mismatch at the on-target site.
Discussion
We report a new vectorized prime editing system capable of correcting the SCD mutation in vitro in CD34+ cells derived from patients with SCD as well as in ex vivo and in vivo HSC gene therapy approaches in a SCD murine model. The in vivo approach is technically simple; it only involves subcutaneous injections to mobilize HSCs, an intravenous injection of a single nonintegrating HDAd-PE5max vector, and expansion of corrected HSPCs by early treatment with low-dose, intraperitoneal O6BG/BCNU. At week 16 after HDAd-PE5max injection, >30% of lineage-negative progenitor colonies contained at least one corrected allele, which is of therapeutic relevance as indicated by allogeneic HSC–transplantation studies with normal or SCD-heterozygous donors in whom chimerism as low as 20% at the HSC level can result in 100% circulating donor RBCs.37,39 In our in vivo editing study, on average 43% of HbS was replaced by HbA in erythrocytes. In vivo prime editing greatly improved the SCD phenotype, with a complete cure in some mice. The editing rate remained stable after transplantation of Lin– BM cells into secondary recipients indicating that the editing occurred in long-term repopulating cells. In vivo prime editing of the SCD mutation was accurate with <1% undesired indels at the target site within the β-globin coding region and no detectable OT editing of concern. The latter could be because of a more stringent requirement for the target sequence to match the pegRNA elements for successful prime editing, when compared with the CRISPR/Cas9 system.8 Clearly, low indel frequency is important to minimize the chance of creating β0 alleles and replace SCD with β-thalassemia.
So far, correction of the SCD mutation, in an ex vivo HSC gene therapy setting, has only been achieved by homology directed repair involving a CRISPR/Cas9–mediated double-stranded DNA break and the delivery of an exogenous DNA repair template in the context of a rAAV vector.40-43 A major efficacy-limiting step in that approach is homologous recombination.44 Furthermore, in vivo delivery of the 2-component system to HSCs will be difficult. Considering that current CRISPR-SpCas9 versions trigger on- and off-target double-stranded DNA breaks that can lead to large genomic deletions and rearrangements, the Cas9/ homology directed repair approach also bears the risk of genotoxicity.45
Although our efficacy and safety data are promising, several issues have to be discussed in detail:
Safety concerns with our approach are related to the IV injection of HDAd5/35++ vectors: the reticuloendothelial system sense HDAd vectors and can trigger the release or production of proinflammatory cytokines. We have developed pharmacological approaches to avoid these responses in mice13 and nonhuman primates (NHPs).26,36,46
G-CSF mobilization triggers leukocytosis and release of proinflammatory cytokines from granulocytes. This would be particularly critical in patients with SCD.47 Recently, we developed a simpler, rapid, and more HSC-selective mobilization regimen using truncated GRO-β (MGTA-145), a CXCR2 agonist, and plerixafor/AMD3100. We demonstrated that it mediates efficient mobilization and in vivo transduction of HSCs with β-thalassemia correction in mice.48 Plerixafor plus small molecule VLA4 antagonists, such as BIO5192 or its improved derivatives (eg, WU106), are also among the alternative G-CSF–free mobilization protocols.48,49 Notably, ex vivo HSC gene therapy approaches for SCD would also benefit from G-CSF–free mobilization regimens.
Potential cytotoxicity from HSC transduction with HDAd5/35++ vectors and expression of the prime editing machinery: in vitro transduction of SCD CD34+ cells with HDAd-PE5max or HDAd-GFP (control vector) did not decrease the number of progenitor colonies compared with untransduced CD34+ cells. The ex vivo study did not indicate an engraftment disadvantage of HDAd-PE5max transduced Lin– cells. This is further corroborated by the efficient return of transduced, mobilized HSCs to the BM in a process in which they compete with nontransduced, mobilized HSCs. Taken together, these data do not indicate cytotoxicity associated with HDAd-PE5max transduction of HSCs.
Central hypotheses of our approach were that episomal HDAd-PE5max vector genomes would be efficiently lost in rapidly dividing cells, whereas they would persist for a prolonged time in slowly dividing HSCs, which would allow for cell expansion by O6BG/BCNU. In support of the first hypothesis, we show that after transduction of CD34+ cells with an HDAd-mgmt/GFP vector and subsequent ED, the percentage of GFP-expressing cells declines almost 2 orders of magnitude, most likely owing to loss of episomal genomes (supplemental Figure 13). This is also expected to occur once HSCs undergo expansion and differentiation. The observation that VCNs in BM MNCs at week 6 were ∼0.03 supports the latter, whereas no vector DNA was detectable in PBMCs. Kinetics studies of VCN and transgene expression in BM LSK cells after in vivo HSC transduction with HDAd-mgmt/GFP support the second hypothesis and justify the O6BG/BCNU treatment regimen on days 6, 19, and 33. During that period, transgene expression declined only 30% to 50% regardless of in vivo selection. A potential advantage of prolonged HDAd-PE5max persistence in HSCs could be more efficient prime editing. However, it also raises concerns, specifically, increased OT site editing, immune responses against heterologous elements of the prime editing machinery (including the bacterial nCas9 and the retroviral RT), and potential tumorigenicity or clonal dominance mediated by a dominant-negative MLH1 protein, (which in its wild-type form is a tumor suppressor). Several observations argue against a detrimental effect of transient dn-MLH1 expression in our mouse study. Analysis of indels as clonal marker did not indicate oligoclonal hematopoiesis. In addition, the indel levels in primary mice and secondary mice demonstrate no significant change (Figure 4J). Considering the PE created low frequency of indels, conclusions about clonal expansion of edited cells should be validated through more robust approaches, such as single cell NGS.
Blood cell counts and lineage composition of BM cells in primary and secondary mice did not show abnormalities. Clearly, PE systems without dn-MLH-1 would be preferable. In this context, we showed in studies with HDAd-PE3max, a vector that does not contain the dn-MLH1 expression cassette, efficient in vivo HSC prime editing at a close to therapeutic level of ∼18% (supplemental Figure 11).
HDAd5/35++ transduction of nonhematopoietic tissues. The target receptor of HDAd5/35++ vectors, CD46, is expressed on all nucleated cells, which raises safety concerns. Previous biodistribution studies in mobilized mice and NHPs injected IV with HDAd5/35++ vectors detected vector genomes in splenic and BM HSCs, and, at a lower level, in those in liver and lungs.36,50 This is in agreement with our early studies in baboons with IV injected first-generation CD46-targeting Ad5/35 and Ad5/11 vectors.50 A potential explanation is that CD46 receptor density and accessibility is not sufficiently high on most nonhematopoietic tissues to allow for efficient viral transduction.13,51 Within the blood system, HDAd5/35++ transduction of BM HSCs was higher than transduction of total BM MNCs cells in hCD46tg and NOG/hCD34 mice, which correlated with higher CD46 levels in HSCs.13
Here, we detected target site editing in tissue lysates of the gastrointestinal tract, kidney, liver, lung, heart, and brain. On the basis of our biodistribution studies after in vivo HSC transduction or selection in mice, it can be assumed that most of the vector DNA signals could have originated from contaminating edited blood cells and/or edited tissue macrophages that originated from transduced HSCs as shown in a recent study with a GFP vector.35 This, however, does not exclude editing in nonhematopoietic tissues. Future studies with purified hepatocyte and lung epithelial cells or microdissected parenchymal cells from transduced animals will assess the level of editing in nonerythroid cells. On the basis of the absence of abnormalities in hematological and histological analyses in the current in vivo prime editing study, we speculate that editing of the βS-globin gene in nonerythroid cells (in which hemoglobin genes are not expressed) would not have critical side effects.
O6BG/BCNU selection is currently a limitation of the approach, specifically in patients with SCD being at increased risk of developing leukemia and clonal hematopoiesis.52,53 Previous in vivo HSC transduction studies with HDAd-GFP vectors showed ∼10% GFP-positive LSK cells13 in mice and ∼7% GFP-positive CD34+/CD45RA–/CD90+ 39 in NHPs in the BM during the first week after transduction (before in vivo selection). Approaches to achieve more efficient transduction and BM homing of mobilized HSCs26 and better survival of transduced mobilized cells54 could reduce or eliminate the need for O6BG/BCNU selection. In addition to strategies that increase the number of edited HSCs in the BM, our current efforts are also focused on alternative in vivo selection approaches, including the use of toxins targeted to receptors on HSCs or on common myeloid progenitors55,56 together with knockout of the corresponding receptor genes in HSCs using base or PEs. Another strategy that potentially can be used to expand edited HSCs is based on epitope engineering of HSC membrane proteins by base or PEs in combination with monoclonal antibodies that target only unedited cells.57
Recently, virus-like particles have been used for in vivo genome editing of murine liver or retina.58 Moreover, nonviral vehicles based on lipid nanoparticles (LNPs) for messenger RNA delivery hold great promise for in vivo HSC genome editing. However, these new approaches also face the hurdles accounted with intravascular administration of viral vectors, namely the unproductive sequestration by reticuloendothelial cells in liver, spleen, lungs, and other organs, which requires high vector doses, and could be associated acute toxicity. Attempts are under way to address these problems, for example by targeting LNPs to HSCs and suppressing transgene expression in the liver59 or by intraosseous delivery of LNPs.60 Although viruses have evolved highly efficient mechanisms for cellular uptake, intracellular trafficking, and nuclear import, LNP-based nucleic acid delivery still needs major improvements. It is likely that the recent success with LNP-based COVID-19 vaccines will greatly accelerate these endeavors.
We believe that the in vivo prime editing approach to correct the SCD mutation, described in this article, could be the basis for further development toward a cost-effective SCD gene therapy that could be used in developing countries. Clearly, the safety of this approach, specifically editing in nonhematopoietic tissues, will have to be carefully evaluated in upcoming long-term NHP studies.
Acknowledgments
The authors thank Linda Y. Mamiya and Dale Whittington for technical support for isoelectric focusing, high-performance liquid chromatography, and mass spectrometry.
The study was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants R01HL128288 (A.L.) and R01HL141781 (A.L.), NIH National Institute of Allergy and Infectious Diseases grant U01 AI142756 (D.R.L.), NIH National Human Genome Research Institute grant RM1 HG009490 (D.R.L.), NIH National Institute of General Medical Sciences grant R35 GM118062 (D.R.L.), by a grant from Ensoma Bio (A.L., H.-P.K.), and by grants from the Bill and Melinda Gates Foundation: INV-017692 (A.L.) and INV-005701 (D.R.L.), and the HHMI (D.R.L.). G.A.N. was supported by a Helen Hay Whitney Postdoctoral Fellowship and K99 award HL163805. P.J.C. and K.A.E. were supported by National Science Foundation graduate research fellowships.
All experiments involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. The University of Washington is an Association for the Assessment and Accreditation of Laboratory Animal Care International–accredited research institution and all live animal work conducted at this university is in accordance with the Office of Laboratory Animal Welfare Public Health Assurance policy, USDA Animal Welfare Act and Regulations, the Guide for the Care and Use of Laboratory Animals and the University of Washington’s Institutional Animal Care and Use Committee (IACUC) policies. The studies were approved by the IACUC of the University of Washington (protocol number 3108-01).
Authorship
Contribution: C.L. provided the conceptual framework for the study; C.L., A.L., A.G., and E.Y. designed the experiments; C.L., A.G., G.A.N., P.J.C., K.A.E., K.P., E.V., S.G., A.K.A., L.H., T.K., and H.W. performed the experiments; D.R.L. and H.-P.K. provided critical comments on the manuscript; and C.L. and A.L. wrote the manuscript.
Conflict-of-interest disclosure: A.L. and H.-P.K. are academic co-founders of Ensoma Therapeutics. H.P.K. is a paid advisor for Ensoma. P.J.C. is currently an employee of Prime Medicine. D.R.L. is a consultant for Prime Medicine, Beam Therapeutics, Pairwise Plants, Chroma Medicine, and Nvelop Therapeutics, companies that use or deliver genome editing or epigenome engineering agents, and owns equity in these companies. The remaining authors declare no competing financial interests.
Correspondence: Chang Li, Division of Medical Genetics, Department of Medicine, University of Washington, Box 357720, Seattle, WA 98195; e-mail: cli1239@uw.edu.
References
Author notes
Next-generation sequencing data have been deposited in the National Center for Biotechnology Information Sequence Read Archive (SRA) with accession code PRJNA869109. This SRA submission was released on 31 December 2022.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement”in accordance with 18 USC section 1734.

![Therapeutic prime editing in CD46/Townes mice after in vivo HSC transduction with HDAd-PE5max. (A) Experimental procedure. Mice (n = 7) were mobilized using G-CSF/AMD3100 and were transduced in vivo with HDAd-PE5max (8 × 1010 vp/mouse = 3.2 × 1012 vp/kg). For in vivo selection, mice were injected with O6-BG (15 mg/kg, intraperitoneal [IP]) 2 times, 30 minutes apart. One hour after the second injection of O6-BG, mice were injected with BCNU (5 mg/kg, IP). For the second and third cycle, BCNU concentrations were increased to 9 mg/kg and 10 mg/kg, respectively. The mice were killed during week 16 for analyses. Lin– cells were isolated from BM and IV injected into lethally irradiated C57BL/6J mice. The secondary transplanted mice were followed for another 16 weeks for the specified terminal point analyses. (B-F) Editing measured using Sanger sequencing of in vivo–transduced mice. (B) Target base editing (sickle repair and silent PAM) in PBMCs. (C) Editing in cells from different tissues at week 16 after in vivo transduction. (D) Editing in different lineage-positive cell subsets and Lin– cells at week 16. CD3+ T cells, CD19+ B cells, Gr1+ granulocytes, Ter-119+ erythroid cells were sorted from BM MCs using flow cytometry. (E) Allelic analysis in single Lin– cell–derived progenitor colonies. Editing was measured at day 11 after plating of transduced Lin– cells. Data from 4 mice with indicated ear tag number are shown. Twelve colonies for each mouse were analyzed. (F) Editing in tissues (n = 4). (G-I) Analyses of secondary recipients. Lin– cells from in vivo–transduced mice were transplanted into lethally irradiated C57BL/6 mice (n = 7) (cells from donor into 1 recipient). (G) Engraftment based on human CD46 expression in PBMCs. (H) Editing measured using Sanger sequencing in PBMCs of secondary recipients at indicated weeks after transplantation. (I) Editing measured using Sanger sequencing at week 16 after transplantation in PBMCs, splenocytes, and BM cells. (J) Target base conversions and indel frequency measured using NGS. Week 16 BM MNCs samples from primary and secondary mice were used. K-N) Analyses of hemoglobin composition. Whole blood samples at week 16 after in vivo (n = 7) and ex vivo (n = 5) transduction were analyzed. Samples from untreated CD46/Townes mice (n = 3) were used as control. (K) Percentages of hemoglobin tetramers as measured using HPLC. Representative chromatograms are shown in supplemental Figure 12A. (L) Percentages of hemoglobin subunits as measured using mass spectrometry. (M) Representative chromatogram pattern showing the separation of βA from βS globin chains. The labeled percentages were calculated based on the peak areas. See supplemental Figure 12B for representative full spectrum chromatograms. (N) Separation of hemoglobin variants using isoelectric focusing electrophoresis. Each lane represents 1 mouse (ear tag number labeled) or AFSC controls. Bands in AFSC controls indicating 4 different hemoglobin variants are labeled. For B-D and F-J, each dot represents 1 animal. Data shown are mean ± standard deviation, wherever applicable. AFSC, hemoglobin A, F, S, and C controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/17/10.1182_blood.2022018252/5/m_blood_bld-2022-018252-gr4.jpeg?Expires=1765893271&Signature=PI-iJYLATF8rQpnG1nfRqcn-0BfQcdFSQrEhgNLy6oSU7-McBW8whsDiy2B5-nEMS4omjcFVHEIvoU8ZOVHI3VHY~zOo7TZOSfH8DhG~rQ3IILoT7ud-pjQEUOZcAewAxuOcCcO5YFZMFLb7G1XbNmeGgBKP-G6RRf3uHv7Q8uIoaDWyYdov94d0AwE6DwvziiN9m5nNOx6pYlaaQIw7VIOP5-u8JDfxV1Fd0~UwnIolrNz3e76~PRKTjijM7JQIi~Pu1awDOfVmldfaprVA3BizNjTKE7FXkTWah46A-02MoLrhLzDTwqWh9D4MBjSPu7Ey6~F4FyNahBD1KImvbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal