In this issue of Blood, Tadmor et al1 demonstrate in a retrospective study the positive impact of antiviral treatment for patients with chronic lymphocytic leukemia (CLL) who test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients included in the study from an Israelian hospital network were positive for SARS-CoV-2 by polymerase chain reaction (PCR). Rapid treatment with the combination of nirmatrelvir plus ritonavir (median of 1 day after the positive PCR test) correlates with a statistically significant lower risk of the composite end point of hospitalization or death as well as a lower risk of death and hospitalization separately, as compared with patients with CLL with PCR-positive SARS-CoV2 but not receiving antiviral therapy (see figure).
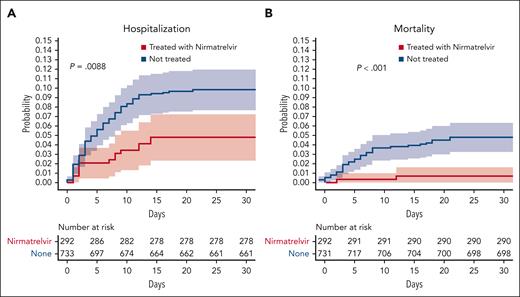
Lower risk of hospitalization (A) and death (B) is correlated with nirmatrelvir plus ritonavir treatment upon a positive SARS-CoV-2 test for patients with CLL. See supplemental Figure 2 in the article by Tadmor et al that begins on page 2239.
Lower risk of hospitalization (A) and death (B) is correlated with nirmatrelvir plus ritonavir treatment upon a positive SARS-CoV-2 test for patients with CLL. See supplemental Figure 2 in the article by Tadmor et al that begins on page 2239.
Another single-institution study recently demonstrated breakthrough COVID-19 infections in the Omicron era among patients with B-cell malignancies despite being fully vaccinated and receiving passive immunization with tixagevimab and cilgavimab.2 Mortality was below 2% for patients with CLL contracting COVID-19 in the Omicron era. However, the subgroup of patients with CLL and close contact with the hospital due to CLL, COVID-19, or comorbidity still had mortality above 20%.3 A population-based assessment of excess mortality among patients with hematological malignancies demonstrated that patients with CLL above 65 years of age were the only subgroup of patients with excess mortality following COVID-19 infections in 2022 during the Omicron era.4
Due to both intrinsic and treatment-induced immune dysfunction, infections remain the major cause of death for patients with CLL.5 First-line targeted CLL treatment with triplet combination therapy (ibrutinib, venetoclax, and obinutuzumab) resulted in similar levels of infections as seen with combination chemoimmunotherapy.6 Additionally, infections cause significant morbidity and the majority of excess deaths after first-line therapy for patients with CLL.7 Comorbidities are frequent among patients with CLL and worsen the risk of death from infections as well as all-cause mortality.8 On this background, the study by Tadmor et al demonstrates that patients only receiving ritonavir, probably due to renal impairment or comorbidity precluding the administration of nirmatrelvir, have a trend for higher risk of hospitalization or death as compared with patients with CLL contracting COVID-19 but not receiving antiviral treatment.
A recent systematic review of interventions to reduce infections in general across patients with hematological malignancies concluded that vaccination and immunoglobulin replacement therapy, but not prophylactic antibiotics, could reduce infections to some degree, although no intervention reduced all-cause mortality.9 Although retrospective, the work by Tadmor et al for the first time demonstrates that an intervention to reduce the severity of the infection by early antiviral treatment upon a positive SARS-CoV-2 test can reduce the risk of hospitalization and death for patients with CLL.
Mortality and morbidity from infections for patients with hematological malignancies in general, and for patients with CLL in particular, need ongoing attention. Clinical trials and retrospective assessment of real-world data are needed to test the efficacy of different prophylactic interventions and treatment approaches to reduce the morbidity and mortality due to immune dysfunction and infections in patients with hematological malignancies and in particular for patients with CLL.
Elderly patients with CLL and other comorbid conditions or heavily pretreated for CLL are at the highest risk of morbidity and mortality from infections. Importantly, Tadmor et al identify this patient population as those with the greatest potential benefit from early treatment with the combination of nirmatrelvir plus ritonavir upon a positive SARS-CoV-2 test. This study highlights the importance of identifying patients with CLL at highest risk of infections for inclusion in trials testing interventions to reduce the risks of infections. As next steps toward identifying such patient populations, machine learning algorithms based on data-driven pattern recognition using real-world data and clinical trial data should be explored.10
Most current guidelines for patients with CLL recommend vaccination against pneumococci, influenza, and COVID-19 along with passive immunization against COVID-19 and immunoglobulin replacement therapy for specific subgroups. Furthermore, prophylaxis toward pneumocystis pneumonia and herpesvirus is recommended in specific treatment situations. These guidelines may now be supplemented with the recommendation of nirmatrelvir plus ritonavir as soon as possible after testing positive for SARS-CoV-2 in CLL patients who are above 65 years of age, are heavily pretreated, have comorbidity associated with increased risk of infections, or are receiving immunoglobulin replacement therapy. Furthermore, optimization of early treatment and prevention of infections in patients with CLL should be tested as next steps toward improving outcomes for patients.
Conflict-of-interest disclosure: The author has received research funding and/or consultancy fees from AstraZeneca, Janssen, AbbVie, Beigene, Genmab, CSL Behring, Octapharma, Takeda, and Novo Nordisk Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal