Key Points
Administering nirmatrelvir for patients with CLL is associated with a lower rate of COVID-19–related hospitalization and mortality.
Patients older than 65, heavily treated (>2 previous treatments), and receiving IVIG prophylactic therapy benefit most from nirmatrelvir.
Abstract
Patients with chronic lymphoid leukemia (CLL), even in the Omicron era and after vaccination, suffer from persistent COVID-19 infection, higher complications, and mortality compared with the general population. In this study, we evaluated retrospectively the effectiveness of nirmatrelvir + ritonavir among 1080 patients with CLL who were infected with severe acute respiratory syndrome coronavirus 2. Nirmatrelvir administration was associated with a reduction in COVID-19–related hospitalization or death by day 35. Specifically, the rate of COVID-19–related hospitalization or death in the treated group compared with the untreated group was 4.8% (14 out of 292) vs 10.2% (75 out of 733), respectively. Moreover, we report a 69% relative risk reduction in COVID-19–related hospitalization or death in patients with CLL at the age of ≥65 years. Multivariate analysis indicates that patients aged >65 years, patients who received heavy treatment (>2 previous treatments), patients with recent hospitalizations, intravenous immunoglobulin (IVIG) treatment, and comorbidity had significant improvement outcomes after treatment with nirmatrelvir.
Introduction
The COVID-19 pandemic had a significant impact on patients with chronic lymphocytic leukemia (CLL) because the disease itself and/or its treatment causes immune dysfunction.1 These patients have a higher hospitalization rate and a greater risk of mortality owing to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.2 The development of vaccination against the virus could provide immune protection to ∼50% to 60% of all patients with CLL, which leaves a considerable percentage of patients unprotected.3-5
Unlike the first variants of the SARS-CoV-2 infection, infection with the Omicron variant has been described as a milder disease in the general population and in patients with hematological malignancies.6 Recently it was reported that patients with CLL also have a lower risk of death from COVID-19 in the Omicron era.7 However, patients with CLL might be at increased risk of developing persistent COVID-19 infection mainly if they were treated with anti-CD20 monoclonal antibodies,8-10 and might be at increased risk of considerable morbidity and mortality.6
The oral protease inhibitor, nirmatrelvir, has demonstrated antiviral activity against all coronaviruses that are known to infect humans. When coadministered with ritonavir, it reduces the incidence of COVID-19–related hospitalization or mortality among patients who are high risk,11 mainly in patients aged >65 years.12 Early drug administration was also reported to be efficacious during the Omicron wave.13 This study aims to retrospectively evaluate the effectiveness of nirmatrelvir + ritonavir among patients with CLL infected with SARS-CoV-2.
Study design
The cohort is based on data obtained from electronic medical records after receiving approval from the ethical committee for members of Maccabi Healthcare Services (MHS), the second-largest healthcare organization in Israel, with ∼2.5 million patients with health insurance.14
We analyzed the outcome of all patients with CLL, diagnosed based on the International Workshop on Chronic Lymphocytic Leukemia (IWCLL) diagnostic criteria.15 These patients were identified using the International Classification of Diseases, Ninth Revision, coding system (204.10-12) which is added to the patient’s medical record after confirmation of the diagnosis by an expert hematologist, and in addition, the diagnosis was recorded in the MHS registry for hematologic neoplasm diseases. As with previous retrospective studies of CLL conducted on the MHS database,16 to ensure the validity of the cohort, the patients had to meet 1 of the following criteria: (i) have received anti-CLL therapy at least once since diagnosis, or (ii) if treatment-naive, have at least 1 complete blood count result indicative of an absolute lymphocyte count above 5 × 109/L at any time during the study.
The study period began on 2 January 2022 (the first day nirmatrelvir was available to patients in Maccabi) and ended on 20 September 2022 (the first day the messenger RNA (mRNA) Omicron booster was administered to patients in Maccabi). During the study period, the Omicron sublineages were dominant in patients in Israel (supplemental Figure 1; available on the Blood website).
The date of any polymerase chain reaction (PCR) tests for SARS-CoV-2, and the result(s) of the test of all MHS members are recorded centrally, including tests performed outside MHS clinics (eg, drive-in/walk-in test centers or in the airport test center). This PCR data were previously used in various COVID-19–related studies.17-19 Because patients with CLL are considered as patients who are at high risk, they were eligible for PCR tests, without the need to perform an antigen test first. During the study period, 6213 PCR tests were performed on patients with CLL, with a positivity rate of 29.3%. If the same patient performed several PCR tests, we used the earliest positive PCR result as the index date. Patients detected as positive during inpatient care were excluded from the analysis. Moreover, 3 patients who tested positive in an antigen test but then tested negative in the subsequent PCR test were excluded from the study. Notably, the eligibility for antiviral therapy requires a positive PCR test result and that <5 days should have passed since the onset of the symptoms. After individual drug approval, it is being delivered on the same day or the following day to the patient’s home.
The aim of the study was to investigate the real-world effectiveness of nirmatrelvir therapy in preventing COVID-19–related hospitalization or death of any cause within 35 days.
Statistical analysis
The Kaplan-Meier method was used to compare the proportion of patients hospitalized owing to SARS-CoV-2 infection or who died of any cause within 35 days among following 3 subcohorts: patients who received nirmatrelvir therapy, patients who received molnupiravir therapy, and patients who did not receive any antiviral therapy.
To determine whether the treatment effect varied across various factors, such as age, sex, etc., we performed a subgroup analysis as suggested by Hammond et al.11 Nominal 95% confidence intervals (CI) are also provided. The standard errors were calculated using Greenwood's formula, followed by a z-test.
Using a multivariate Cox proportional–hazards regression model, the association between nirmatrelvir therapy and COVID-19 outcomes was estimated with the adjustments to covariates (Table 1). Given that there are many potential confounders, we applied a 2-step test for selecting the relevant covariates, as suggested by Arbel et al.12 Schoenfeld’s global test was applied to the survival curves to test the proportional-hazards assumption for those variables. Finally, a multivariate Cox proportional–hazards regression model was used to estimate the association among each covariate that met the 2 above mentioned testing criteria (Table 1). All statistical analyses were performed using the R statistical software 4.2.1 (2022-06-23) (Foundation for Statistical Computing, Vienna, Austria).
The association between nirmatrelvir therapy and COVID-19–related hospitalization or death was estimated with the use of a multivariate Cox proportional–hazards regression model after adjustment for confounding factors
| Variable . | HR for COVID-19–related hospitalization or death . | P value . |
|---|---|---|
| Age | 1.05 (1.03-1.07) | <.001∗ |
| Number of previous CLL treatments | 1.39 (1.17-1.65) | <.001∗ |
| Recent hospitalizations† | 1.92 (1.25-2.95) | .0027‡ |
| IVIG treatment | 1.96 (1.2-3.4) | .019§ |
| Nirmatrelvir | 0.48 (0.13-0.63) | .002‡ |
| Previous COVID-19 infection | 0.22 (0.07-0.72) | .0199§ |
| Doses of COVID-19 vaccine|| before infection | 0.75 (0.63-0.89) | <.001∗ |
| Recent melanoma† | 1.79 (1.09-2.94) | .0213§ |
| Recent other malignancy† | 0.42 (0.19-0.91) | .0284§ |
| Asthma | 2.66 (1.22-5.81) | .0141§ |
| Myeloproliferative diseases | 3.19 (1.58-6.43) | .0012‡ |
| Chronic kidney disease status | 1.1 (0.62-1.95) | .667 |
| Variable . | HR for COVID-19–related hospitalization or death . | P value . |
|---|---|---|
| Age | 1.05 (1.03-1.07) | <.001∗ |
| Number of previous CLL treatments | 1.39 (1.17-1.65) | <.001∗ |
| Recent hospitalizations† | 1.92 (1.25-2.95) | .0027‡ |
| IVIG treatment | 1.96 (1.2-3.4) | .019§ |
| Nirmatrelvir | 0.48 (0.13-0.63) | .002‡ |
| Previous COVID-19 infection | 0.22 (0.07-0.72) | .0199§ |
| Doses of COVID-19 vaccine|| before infection | 0.75 (0.63-0.89) | <.001∗ |
| Recent melanoma† | 1.79 (1.09-2.94) | .0213§ |
| Recent other malignancy† | 0.42 (0.19-0.91) | .0284§ |
| Asthma | 2.66 (1.22-5.81) | .0141§ |
| Myeloproliferative diseases | 3.19 (1.58-6.43) | .0012‡ |
| Chronic kidney disease status | 1.1 (0.62-1.95) | .667 |
Variables that met the testing criteria and were significantly associated with the outcome served as the inputs for the multivariate regression analysis. IVIG, intravenous immunoglobulin.
Very highly statistically significant at the level 0.001
Recent refers to last 3 years.
Highly statistically significant at the level 0.01
Statistically significant at the level 0.05
Number of doses counts both messenger RNA–based vaccines (mainly Pfizer) and passive vaccines (75 patients received tixagevimab and cilgavimab).
The study was approved by the Helsinki committee of the Maccabi health system (approval number 134-21).
We received an exemption from informed consent because of the nature of this retrospective, anonymous big-data study.
Results and discussion
During the study period, 2929 active patients with CLL were reported in the MHS database. The study cohort comprised all patients in Maccabi with CLL aged >18 years and had confirmed outpatient SARS-CoV-2 infection with PCR test during the study period (1080 patients in total). Patient characteristics are summarized in supplemental Table 1; 65.5% were male with a median age of 71 years. Most of the patients (95%) received at least one shot of the vaccine, 94% received at least 2 shots, and 54% received at least 4 shots. BNT162b mRNA was the most administrated vaccine (>99%). Moreover, 82 patients (7.6%) received at least 1 dose of tixagevimab + cilgavimab before infection. All patients in the cohort were eligible to receive nirmatrelvir therapy, because they were assessed as being at high risk for progression to severe disease. The median time (interquartile range) to treatment with nirmatrelvir was 1 day (1-1) and the average time to treatment was 0.94 days, in which 22.6% of the patients who were treated received nirmatrelvir on the same day they obtained a positive SARS-CoV-2 PCR result, and 63% of the patients received nirmatrelvir on the subsequent day. Eleven percent of the patients received nirmatrelvir after 2 days, 1.6% after 3 days, and <1% after 4 days.
Two hundred ninety-two patients were treated with nirmatrelvir, out of which 13 needed COVID-19–related hospitalization (4.4%), 1 patient died during hospitalization, and another patient treated with nirmatrelvir passed away before being hospitalized. Thus, in total 14 patients (4.8%) who underwent the nirmatrelvir therapy met the criterion for an event.
Supplemental Figure 2A-B presents the cumulative percentage of patients with COVID-19–related hospitalization and death of any cause estimated using the Kaplan-Meier model. Treatment with nirmatrelvir is associated with a lower hospitalization rate (P = .0088) and mortality rate (P < .001).
If nirmatrelvir could not be administered owing to drug interaction, comorbidity, or drug availability, treatment with molnupiravir was offered as of 15 January 2022. Sixty-two patients received molnupiravir, 9 requiring hospitalization (14.5%). Patients treated with molnupiravir were more likely to have been hospitalized recently (P = .03) and to have been previously treated for CLL (P = .019) than were those treated with nirmatrelvir (supplemental Table 1). Moreover, patients treated with molnupiravir tend to be in a more advanced chronic kidney disease stage than were patients treated with nirmatrelvir (P = .008). In addition, 44 patients who were not administered any antiviral drug required hospitalization (6.1%).
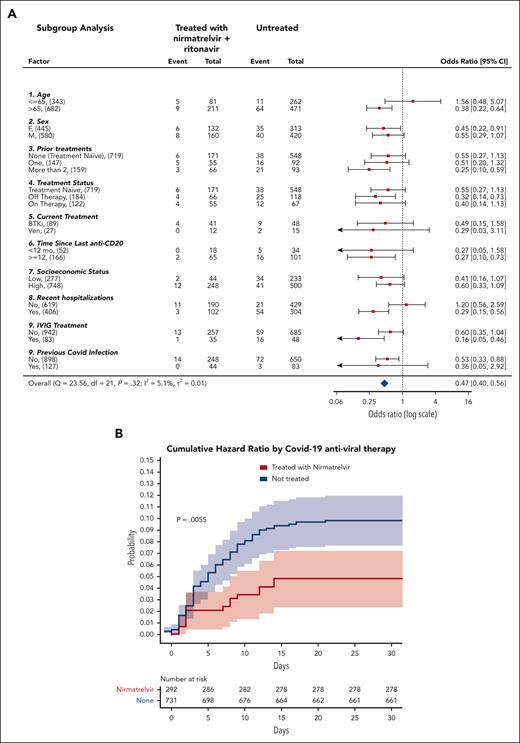
Results from subgroup analyses (Figure 1A) were statistically significant (P < .05) for older patients (aged > 65 years), patients who were heavily treated for CLL (at least 2 CLL treatments), patients who received anti-CD20 >1 year ago, patients who were recently been hospitalized, patients who received intravenous immunoglobulin (IVIG) treatment any time in the past, and were not previously infected by SARS-CoV-2. Nirmatrelvir was ineffective in younger patients (aged ≤65 years), as previously seen12 or in patients who had recently recovered from SARS-CoV-2 infection.
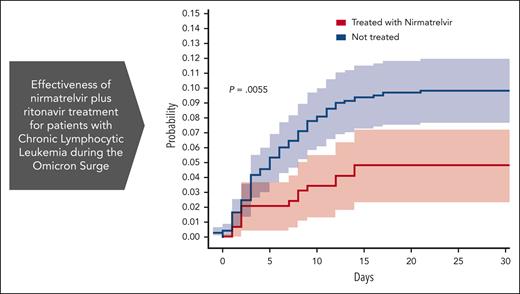
Analysis of the effectiveness of nirmatrelvir plus ritonavir. (A) Subgroup analysis of the differences of the proportions of patients treated within 5 days of positive PCR who had COVID-19–related hospitalization or death from any cause through day 35. (B) Cumulative HR by COVID-19 antiviral therapy. For patients who received treatment with antiviral agents, time zero corresponded to the time at which a patient began the treatment. For patients who did not receive treatment with either nirmatrelvir or molnupiravir, time zero corresponded to the time at which each patient had received a positive COVID-19 PCR. Setting time zero to the time at which each patient had received a positive COVID-19 PCR for both patients who received and did not receive treatment, yields similar results (supplemental Figure 3). Cumulative HR curve disregards 2 patients who did not receive treatment for whom the exact event time could not be determined. The shaded areas indicate the 95% CI.
Analysis of the effectiveness of nirmatrelvir plus ritonavir. (A) Subgroup analysis of the differences of the proportions of patients treated within 5 days of positive PCR who had COVID-19–related hospitalization or death from any cause through day 35. (B) Cumulative HR by COVID-19 antiviral therapy. For patients who received treatment with antiviral agents, time zero corresponded to the time at which a patient began the treatment. For patients who did not receive treatment with either nirmatrelvir or molnupiravir, time zero corresponded to the time at which each patient had received a positive COVID-19 PCR. Setting time zero to the time at which each patient had received a positive COVID-19 PCR for both patients who received and did not receive treatment, yields similar results (supplemental Figure 3). Cumulative HR curve disregards 2 patients who did not receive treatment for whom the exact event time could not be determined. The shaded areas indicate the 95% CI.
The association between nirmatrelvir therapy and COVID-19–related hospitalization or death was also tested after adjustment for confounding factors. According to the multivariate Cox proportional–hazards regression model presented in Table 1, significantly (P = .018) fewer recipients of nirmatrelvir had COVID-19–related hospitalization or death (14/292 patients [4.8%]) than those who received no treatment (75/733 [10.2%]). This represents a 53% relative risk reduction in COVID-19–related hospitalization or death from any cause (Figure 1B). Because the number of patients who received molnupiravir was relatively small, and many of them had severe contradictions (eg, severe renal impairment), the corresponding Kaplan-Meier curve has an extensive CI, and it does not allow any conclusion to be drawn concerning the effectiveness of molnupiravir (supplemental Figure 4). Thus, for the sake of clarity, we excluded them from the results presented in Figure 1.
In addition to nirmatrelvir therapy, the following factors were also found to be significantly associated with the adverse outcome according to the multivariate Cox proportional–hazards regression model: age, number of previous CLL treatments, recent hospitalization, IVIG treatment, asthma, and myeloproliferative diseases (refer to Table 1 for P values).
Seven hundred forty-six patients with CLL aged ≥65 years have been included in the cohort; of these, 217 have been treated with nirmatrelvir, 9 of whom required hospitalization (4.1%). Fifty-one patients were treated with molnupiravir, of whom 9 required hospitalization (17.6%), and 36 out of the remaining patients (7.5%) also needed hospitalization. The hazard ratio (HR) for COVID-19–related hospitalization (disregarding death) for older patients with CLL (aged ≥65 years) who received nirmatrelvir therapy is 0.55. A recent retrospective study performed in Israel on a general population which is at high risk12 has shown that nirmatrelvir therapy leads to a HR of 0.27 for hospitalization among older patients. Thus, it is possible that in patients with CLL, nirmatrelvir therapy is not as effective as in the general population which is at high risk. In conclusion, the use of nirmatrelvir for patients aged ≥65 years with CLL and SARS-CoV-2 infection is associated with a lower rate of hospitalization and mortality.
We compared the treated group (molnupiravir or nirmatrelvir) with the untreated group and concluded that the differences are not statistically significant with P = .061 (supplemental Figure 5). Notably, although nirmatrelvir was found to be statistically significant for reducing HR (Table 1 and supplemental Table 2), molnupiravir monotherapy was not found to be statistically significant (P = .1655). However, these results should be taken cautiously owing to the relatively small number of patients treated with molnupiravir monotherapy. A retrospective study20 performed in Israel during the Omicron surge has shown that in patients who are at high risk and aged ≥65 years, a molnupiravir monotherapy yields an adjusted HR of 0.55 (95% CI, 0.34-0.88) for COVID-19–related hospitalization. The same team also analyzed the effectiveness of nirmatrelvir12 and reported an adjusted HR of 0.27 (95% CI, 0.15-0.49) for COVID-19–related hospitalization among patients who are at high risk and aged ≥65 years. Thus, in the general high-risk Israeli population, the reported effectiveness of molnupiravir monotherapy is not as prominent as in the nirmatrelvir-based therapy.
Our study has several limitations. Because this is a retrospective cohort study, various confounders may have contributed to the biasness in the observed results. Specifically, unmeasured confounding might exist, such as access to antiviral therapy. Although patients with CLL were eligible for antiviral treatment, only a small portion of the patients received either nirmatrelvir or molnupiravir. It is unknown why the remaining patients with CLL did not receive antiviral therapy.
Acknowledgment
This work was supported by a grant from KSM Maccabi's Research and Innovation Center.
Authorship
Contribution: T.T. designed, organized, and wrote the manuscript; H.A. prepared the data and preprocessed the data; and L.R. performed the statistical analysis and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lior Rokach, Department of Software and Information Systems Engineering, Ben-Gurion University of the Negev, P.O.B. 653, Beer-Sheva, Israel 8410501; e-mail: liorrk@bgu.ac.il.
References
Author notes
Data are available on request from the corresponding author, Lior Rokach (liorrk@bgu.ac.il).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal