An important role for cytotoxic T cells was demonstrated many years ago in patients with chronic immune thrombocytopenia (ITP), yet how CD8+ T cells evolve and perpetuate chronic ITP has been less well studied. In this issue of Blood, Malik et al1 report that patients with chronic ITP have clonal expansions of a particular subset of CD8 T cells known as terminally differentiated effector memory (TEMRA) T cells. These cells persisted over many years, were more evident in refractory ITP, and were more prevalent when the platelet count was low.
ITP is an autoimmune bleeding disease with a complex pathophysiology involving both antibody-mediated platelet clearance as well as an important contribution by T cells. CD4+ T cells are considered critical for initiating and maintaining the disease, and CD8+ T cells are implicated in contributing to the destruction of platelets and/or megakaryocytes (MKs).2,3 Unfortunately, there are no biomarkers that can help in the diagnosis of chronic ITP or help select the best treatment options for patients. A better understanding of the pathophysiology of the disease, particularly with respect to CD8+ T cells, is needed.
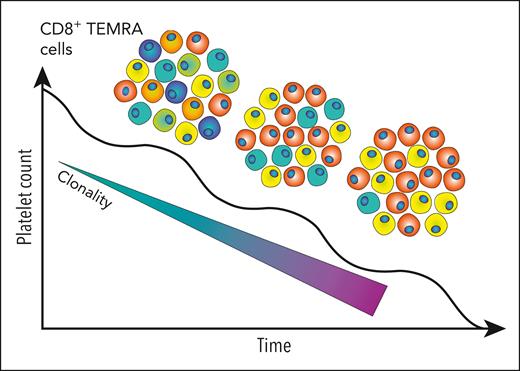
Malik et al examined peripheral blood CD8+ T-cell subsets from 32 patients with chronic ITP and categorized them into naïve T cells and several subsets of memory T cells including central memory T cells, effector memory T cells, and TEMRA T cells. The authors convincingly demonstrate that among these CD8+ T-cell memory subsets, TEMRA cells were present at significantly higher numbers in patients with ITP vs control subjects, and these TEMRA cells display characteristics of activation with no evidence of T-cell exhaustion as measured by markers. Performing T-cell receptor sequencing on single T-cell clones demonstrated an expansion of TEMRA cells with reduced T-cell receptor repertoire diversity, yet with no overrepresentation of any particular T-cell receptor (TCR) Vβ family (see figure).
In patients with chronic refractory ITP, a fall in platelet count was associated with an increase in T-cell receptor clonality. Representative clones in the CD8+ TEMRA subset with each unique color correspond to unique TCR clones. Correspondingly, the diversity of the repertoire falls as individual clones expand. Figure provided by Michelle M. H. Tan.
In patients with chronic refractory ITP, a fall in platelet count was associated with an increase in T-cell receptor clonality. Representative clones in the CD8+ TEMRA subset with each unique color correspond to unique TCR clones. Correspondingly, the diversity of the repertoire falls as individual clones expand. Figure provided by Michelle M. H. Tan.
Interestingly, patients with more refractory disease had a greater expansion of T-cell clones. Reduced T-cell repertoire diversity was observed in a small number of patients studied longitudinally. One of the most interesting findings was that TEMRA cells were inversely correlated with platelet counts. In coculture experiments with platelets under flow conditions, it was demonstrated that the CD8+ T-cell fraction from patients with ITP promoted platelet activation and apoptosis as surrogates for lysis. Although they did not specifically look at TEMRA cells in the coculture assays, it is most likely that the function of these TEMRA cells was involvement with platelet or MK lysis.
The identification of CD8+ TEMRA cells in chronic ITP is of interest as these terminally differentiated memory cells would have cytotoxic ability, as well as the ability to expand and mount an attack against platelets and MKs. Indeed, animal experiments have demonstrated that platelets and MKs can present antigens to CD8+ T cells.4,5 Further, the observation that they were more prevalent in patients with refractory disease suggests they may be a therapeutic target.
Are these TEMRA cells involved in the actual destruction of platelets/MKs? Or are they present due to the underlying chronic autoimmune disease? Are they playing some other role in patients with ITP? One of the intriguing observations with CD8+ TEMRA cells is that they have been found in the peripheral blood (and cerebrospinal fluid) from patients diagnosed with neurocognitive disorders.6,7 In an earlier study, Cooper et al8 demonstrated the presence of cerebral microbleeds in a high proportion of ITP patients with lower platelet counts, and these microbleeds were associated with longer disease duration. As neurocognitive impairment can also sometimes be found in ITP, it raises the question, what exactly are these TEMRA cells doing?
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal