When the Food and Drug Administration (FDA) approved the first 2 chimeric antigen receptor T-cell therapy (CAR-T) products in 2017, the agency deemed that both required risk evaluation management strategies because of significant toxicities including cytokine release syndrome (CRS) and immune cell–associated neurological syndrome (ICANS). Several articles were published at that time, developing grading systems and standards for administration of immune effector cells and providing guidance on management of these toxicities.1-5 Since then, 4 additional CAR-T products have been approved by the FDA6 (Table 1), all with risk evaluation management strategies requirements, and there are many more in clinical development. A significant amount of experience has been obtained from real-world use of these products in which the patient populations may have more comorbidities than those treated in clinical trials.6-8
Approved CAR-T products in the United States as of March 2023
| Generic name . | Trade name . | Target . | Indication . |
|---|---|---|---|
| Tisagenlecleucel | Kymriah | CD19 | B-cell acute lymphoblastic leukemia among those aged <26 with refractory or multiply relapsed disease. Adult patients with relapsed or refractory large B-cell or follicular lymphoma after ≥2 lines of therapy. |
| Axicabtagene ciloleucel | Yescarta | CD19 | Adult patients with relapsed or refractory large B-cell or follicular lymphoma after ≥2 lines of therapy. Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy. |
| Lisocabtagene maraleucel | Breyanzi | CD19 | Adult patients with relapsed or refractory large B-cell lymphoma after ≥2 lines of therapy or that is refractory to first-line therapy or that relapses within 12 mo of first-line therapy. |
| Brexucabtagene autoleucel | Tecartus | CD19 | Adult patients with relapsed or refractory mantle-cell lymphoma. Adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. |
| Idecabtagene vicleucel | Abecma | BCMA | Relapsed or refractory multiple myeloma after ≥4 prior lines of therapy. |
| Ciltacabtagene autoleucel | Carvykti | BCMA | Relapsed or refractory multiple myeloma after ≥4 prior lines of therapy. |
| Generic name . | Trade name . | Target . | Indication . |
|---|---|---|---|
| Tisagenlecleucel | Kymriah | CD19 | B-cell acute lymphoblastic leukemia among those aged <26 with refractory or multiply relapsed disease. Adult patients with relapsed or refractory large B-cell or follicular lymphoma after ≥2 lines of therapy. |
| Axicabtagene ciloleucel | Yescarta | CD19 | Adult patients with relapsed or refractory large B-cell or follicular lymphoma after ≥2 lines of therapy. Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy. |
| Lisocabtagene maraleucel | Breyanzi | CD19 | Adult patients with relapsed or refractory large B-cell lymphoma after ≥2 lines of therapy or that is refractory to first-line therapy or that relapses within 12 mo of first-line therapy. |
| Brexucabtagene autoleucel | Tecartus | CD19 | Adult patients with relapsed or refractory mantle-cell lymphoma. Adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. |
| Idecabtagene vicleucel | Abecma | BCMA | Relapsed or refractory multiple myeloma after ≥4 prior lines of therapy. |
| Ciltacabtagene autoleucel | Carvykti | BCMA | Relapsed or refractory multiple myeloma after ≥4 prior lines of therapy. |
BCMA, B-cell maturation antigen.
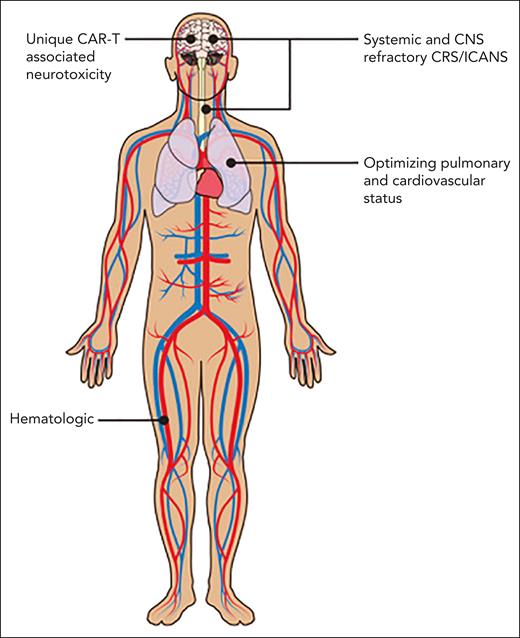
The American Society of Hematology Subcommittee on Emerging Gene and Cell Therapies therefore felt it was timely to develop a How I Treat series focusing on management of complications associated with CAR-T. The articles in this series discuss several types of complications (Figure 1), review the current understanding of etiologies, and provide guidance on therapies.
Michael D. Jain, Melody Smith, and Nirali N. Shah, “How I treat refractory CRS and ICANS after CAR T-cell therapy”
Bianca D. Santomasso, Juliane Gust, and Fabiana Perna, “How I treat unique and difficult-to-manage cases of CAR T-cell therapy–associated neurotoxicity”
Cristina Gutierrez, Tomas G. Neilan, and Natalie S. Grover, “How I approach optimization of patients at risk of cardiac and pulmonary complications after CAR T-cell therapy”
Tania Jain, Timothy S. Olson, and Frederick L. Locke, “How I treat cytopenias after CAR T-cell therapy”
CAR-T toxicities affect many systems. Professional illustration by Patrick Lane, ScEYEnce Studios.
CAR-T toxicities affect many systems. Professional illustration by Patrick Lane, ScEYEnce Studios.
Although the incidence of severe CRS and ICANS has decreased with broader use of preemptive or earlier interventions, persistent or progressive CRS and ICANS remain significant complications of CAR-T. M. D. Jain et al review risk factors and discuss their approach to managing refractory toxicities that persist or progress despite standard treatment with tocilizumab or corticosteroids.
Santomasso et al address the issue of atypical CAR-associated neurotoxicities presenting 3 cases of unique types of neurotoxicity after treatment with CAR T cells and outline their approaches to evaluation and management. These 3 cases illustrate challenging clinical scenarios, including the management of patients with preexisting neurological problems, central nervous system leukemia/lymphoma, or a history of radiation therapy, and the diagnosis and management of delayed CAR-T neurotoxicity.
With expanded use of CAR T cells in real-world settings, many patients have preexisting cardiac and pulmonary comorbidities that would have excluded them from the registration trials. In the article by Gutierrez et al, the authors illustrate how they assess patients with cardiac and pulmonary risk factors, minimize risks, and manage cardiac and pulmonary complications after treatment.
Significant cytopenias may occur in up to 30% of CAR-T recipients. Although an association between elevation of proinflammatory cytokines after CAR-T therapy and delayed bone marrow recovery has been established, the precise underlying mechanisms are still being explored.9 T. Jain et al review the clinical presentation, approach to evaluation, and treatment options for prolonged cytopenias.
A common theme in all 4 articles is the need for more research to better define the etiology of these complications to guide treatment. Nevertheless, these articles provide a framework for the management of complications after CAR-Ts that should prove helpful for the clinical teams caring for these patients.
Conflict-of-interest disclosure: H.E.H. has equity in Allovir and Marker Therapeutics and share options in Fresh Wind Biotherapeutics and Coregen, has served on advisory boards for Tessa Therapeutics and GSK, and has received research support from Athenex and Tessa Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal