Abstract
The clinical use of chimeric antigen receptor (CAR) T-cell therapy is growing rapidly because of the expanding indications for standard-of-care treatment and the development of new investigational products. The establishment of consensus diagnostic criteria for cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS), alongside the steady use of both tocilizumab and corticosteroids for treatment, have been essential in facilitating the widespread use. Preemptive interventions to prevent more severe toxicities have improved safety, facilitating CAR T-cell therapy in medically frail populations and in those at high risk of severe CRS/ICANS. Nonetheless, the development of persistent or progressive CRS and ICANS remains problematic because it impairs patient outcomes and is challenging to treat. In this case-based discussion, we highlight a series of cases of CRS and/or ICANS refractory to front-line interventions. We discuss our approach to managing refractory toxicities that persist or progress beyond initial tocilizumab or corticosteroid administration, delineate risk factors for severe toxicities, highlight the emerging use of anakinra, and review mitigation strategies and supportive care measures to improve outcomes in patients who develop these refractory toxicities.
Introduction
From the earliest experiences, it was clear that chimeric antigen receptor (CAR) T-cell therapy is potent and that toxicity mitigation would be necessary to facilitate widespread use. Indeed, alongside the first approvals for CAR T cells, there was the concurrent approval of tocilizumab, an antibody against the interleukin-6 (IL-6) receptor, to treat cytokine release syndrome (CRS).1 With time, improved recognition of toxicity profiles across various CD19-targeted CAR T-cell constructs facilitated the development of a consensus definition and grading system for CRS and immune effector cell–associated neurotoxicity syndrome (ICANS).2 Entering this second decade of CAR T-cell therapy with more experience with antigen targeting across a breadth of novel constructs, we are better armed to prevent and manage CAR T-cell–associated inflammatory toxicities. Despite these advances, severe toxicities, including refractoriness to standard management with tocilizumab and steroids, still occur.
Initially, use of tocilizumab and/or corticosteroids had been reserved for the treatment of severe CRS and/or ICANS. Now, however, there is an increasing use of these therapies at earlier stages to reduce the incidence of severe CRS and/or ICANS. Strategies include pre-emptive tocilizumab and/or steroids with lower grade toxicity to prevent onset of more severe manifestations,3-5 or even prophylactic treatment before any toxicity is observed.6 These strategies appear to be more effective at reducing CRS severity than ICANS, and there remains caution because of the concern that these interventions could affect longer-term CAR T-cell efficacy.4,7,8 With available strategies, there has been a paradigm shift toward prevention of ≥grade 3 CRS and ICANS.
Although tocilizumab and steroids are first-line interventions for prevention and treatment of CRS and ICANS, respectively,9,10 data for outlining the treatment of refractory CRS and/or ICANS, defined, for the purpose of this manuscript, as persistent or progressive disease despite optimal use of tocilizumab and/or steroids, are lacking. Through a series of cases across the age and disease spectrum, we highlight (1) our approach for considerations and management of toxicities that persist or progress beyond front-line interventions; (2) strategies to identify patients who are at high risk of severe CRS and to consider risk mitigation; and (3) supportive care measures to be optimized among patients receiving increased immunosuppression.
Case 1
A 59-year-old male with diffuse large B-cell lymphoma (DLBCL) proceeded to CD19 CAR T-cell therapy with a bulky abdominal mass (>15 cm) after 3 prior lines of therapy. On day 0, he received tisagenlecleucel and developed grade 1 CRS (fever) on the same day. By day 3, he had progressed to grade 2 CRS with hypoxia and received tocilizumab at 8 mg/kg. Because of persistent fevers and worsening hypoxia, a second dose of tocilizumab was administered 8 hours after the first dose, and dexamethasone, 10 mg IV every 6 hours, was initiated. By day 4, ferritin continued to rise rapidly, tripling within 24 hours to 23 000 ng/mL, and he required transfer to the intensive care unit (ICU) for high-flow oxygen and vasopressors. He developed disseminated intravascular coagulation with low fibrinogen, thrombocytopenia, and acute kidney injury with serum creatinine ≥3 mg/dL. The corticosteroid dose was increased to methylprednisolone 1 g IV daily.
Patients with high tumor burden and primary refractory disease are at high risk of both CRS and lymphoma relapse.11,12 Comparing CD19 CAR T-cell products in DLBCL, CARs incorporating 4-1BB (ie, tisagenlecleucel) have lower toxicity rates than products costimulated by CD28.13,14 Yet, severe CRS has been reported for all products. In patients at high risk or patients requiring repeat tocilizumab doses for lower grade CRS, management commonly incorporates ≥1 doses of dexamethasone 10 mg IV or orally (or, in children ∼1 mg/kg methylprednisolone equivalent dosing). With persistent grade 2 CRS despite repeat doses of tocilizumab and/or single dose corticosteroids (such as in the patient in case 1) or with progression to grade 3 CRS, dexamethasone is often escalated up to 10 mg IV every 6 hours (equivalent dosing of ∼213 mg daily methylprednisolone). With grade 4 CRS, package inserts for commercially available constructs15-17 routinely recommend methylprednisolone 1 g IV per day and/or initiation of alternative CRS-directed therapies. Once a patient has received 2 doses of tocilizumab, repeat doses are generally not recommended, and consideration of both alternative etiologies and incorporation of additional therapies is imperative. Despite tocilizumab and escalating corticosteroids, this patient’s condition continued to deteriorate, prompting additional investigations to aid in the optimal management of refractory CRS.
Case 1 (continued)
Concurrent infections were identified, including bacteremia with Staphylococcus epidermidis and a reactivation of cytomegalovirus (CMV) viremia (840 IU/mL); therefore, antibiotics and CMV treatment were initiated. Early restaging via abdominal computed tomography (CT) scan to check for progressive disease as a cause of refractory CRS showed stabilization of the abdominal mass. On the afternoon of day 4, he was then started on anakinra 100 mg IV every 6 hours for refractory CRS. The patient’s condition subsequently improved. Anakinra and corticosteroid doses were tapered over 1 week. He experienced a maximum ICANS of grade 1. His day 30 positron emission tomography/CT scan showed a complete metabolic remission.
When a patient has clinical deterioration early after CAR T-cell therapy, the top differential diagnoses include refractory CRS, infection, and cancer progression. Infectious workup in this case included standard cultures performed at first fever and every 24 to 48 hours while the patient was persistently febrile as well as tests performed upon clinical deterioration, including evaluation for CMV/adenoviremia and other viremias, nasopharyngeal viral testing for respiratory infections, and chest and abdomen imaging to check for occult infection or disease progression. In this case, neither infection nor cancer progression explained the severity of the clinical presentation. Similarly, the patient had no evidence of gastrointestinal perforation or ischemia, which can occur among patients with abdominal lymphoma.18 For CRS refractory to high doses of corticosteroids (such as dexamethasone 10 mg IV, every 6 hours for 24 hours), the use of the IL-1 receptor antagonist, anakinra, is often considered and will be discussed in later sections. Once a clinical response is achieved, anakinra and corticosteroids can be tapered as clinically indicated, and prolonged use at high doses is generally not indicated. Extra vigilance and enhanced antimicrobial prophylaxis is warranted, given the heightened infectious risks in patients treated with these immunosuppressive therapies,19,20 who may be further compromised with concurrent cytopenias. Although cytopenias are a complication of, and can further complicate, refractory CRS, this topic is discussed in more detail in an accompanying “How I Treat” article.21
The optimal management of CMV reactivation is unknown, and most centers follow hematopoietic cell transplant guidelines.22,23 Close monitoring of organ function during severe CRS is also recommended. Notably, a rapid increase of lactic acidosis and/or development of acute kidney injury may necessitate urgent hemodialysis. Similarly, severe CRS may cause unstable arrhythmias or a sudden drop in ejection fraction.24
Case 2
A 71-year-old female with follicular lymphoma transformed to DLBCL was referred for receipt of CD19 CAR T cells for treatment of progressive nodal and extranodal disease refractory to 3 prior lines of therapy. She was not given any bridging therapy before axicabtagene ciloleucel. On day 2, she developed grade 1 CRS, with persistent fever until day 5, when she was given dexamethasone 10 mg IV once. On day 6, she presented with grade 2 CRS and grade 1 ICANS characterized by fever, hypoxia, and dysphasia, for which she received tocilizumab 8 mg/kg and dexamethasone 10 mg IV once.
In older patients or those who are medically frail who receive CAR T-cell therapy, it is imperative to treat toxicity promptly and closely monitor for clinical worsening. In addition, clinicians should consider early intervention for patients at high risk of developing severe CRS/ICANS, including patients with bulky disease or a high tumor burden. Early intervention with tocilizumab and/or corticosteroids at lower CRS and ICANS grades, and other toxicity reduction strategies, have been reported from nonrandomized clinical trials (Table 1). In cohort 4 of the ZUMA-1 study, patients with DLBCL treated with axicabtagene ciloleucel received early intervention at lower grade toxicities, including an IV dose of tocilizumab and/or dexamethasone 10 mg after 3 days of persistent of grade 1 CRS.31 Although the rates of severe CRS and ICANS in cohort 4 appeared lower than those observed in cohorts 1 and 2 of the ZUMA-1 study, this was confounded by the fact that the baseline tumor burden was much higher in cohorts 1 and 2 than that in cohort 4. Propensity score matching revealed that early intervention was associated with lower rates of severe toxicity; however, this analysis was limited to matching known factors and might not have balanced all the differences between cohorts. It was also found that earlier intervention with corticosteroids may help to limit a more prolonged course of corticosteroids. For example, the comparison of cohorts 1 and 2 with cohort 4 found that the median overall corticosteroid exposure declined from 6886 mg to 939 mg with earlier use of corticosteroids.31 Overall, the trend in clinical practice is toward intervening earlier at lower grades of CRS and ICANS. For elderly patients, there are limited outcome data after CAR T-cell therapy. Ram et al compared clinical outcomes in geriatric patients (n = 41; mean patient age, 76 years) with those in younger patients (n = 41; mean patient age, 55 years).34 The study showed no significant differences in grade ≥3 CRS, grade ≥3 ICANS, hospitalization duration, progression-free survival, or overall survival. These results, as well as findings from other studies, suggest that age alone is not a driving factor for toxicity severity.35-37 However, in patients who may not tolerate prolonged low-grade CRS (such as elderly patients or those who are frail) or patients who are at high risk of subsequent progression to severe toxicities, we recommend early intervention with tocilizumab and/or corticosteroids during lower grade toxicities. Although results of studies incorporating prophylactic corticosteroids are promising,28 additional studies are needed before systematically incorporating the prophylactic use of steroids for risk mitigation.
Select strategies to prevent severe CRS and ICANS
| Strategy . | Disease/product . | Outcome . | Comparison∗ . | Comments . | Reference . |
|---|---|---|---|---|---|
| Fractionated CAR T-cell dosing | |||||
| Fractionated dosing: day 1 (10% dose), day 2 (30%), and day 3 (60%), with day 2 and day 3 doses allowed to be held for early CRS. | Adult B-ALL treated with CD19 CAR T cells. | Fractionated dose: grade ≥4 CRS, 5% (Penn grading scale) Grade ≥3 neurotoxicity, 6%. | High fixed dose: grade ≥4 CRS, 50%; 3 of 6 patients died. | Difficult to implement with fixed-dose commercial CAR T-cell products. | Frey et al25 NCT02030847 |
| Prophylaxis | |||||
| Prophylactic tocilizumab given on day 2. | Adult DLBCL treated with axi-cel. | Prophy toci: grade ≥3 CRS, 3%. Grade ≥3 ICANS, 41%. One case of cerebral edema. | No prophy toci (ZUMA-1 cohorts 1-2)26: grade ≥3 CRS, 13%. Grade ≥3 ICANS, 28%. | Peak IL-6 levels were higher in the prophy toci group, possibly because IL-6R antagonists increase free IL-6. | Locke et al (ZUMA-1 cohort 3)27 NCT02348216 |
| Prophylactic dexamethasone 10 mg on days 0, 1, and 2. | Adult DLBCL treated with axi-cel. | Prophy dex: grade ≥3 CRS, 0%. Grade ≥3 ICANS, 13%. | No prophy dex (ZUMA-1 cohorts 1-2): grade ≥3 CRS, 13%. Grade ≥3 ICANS, 28%. | Lower baseline tumor burden than ZUMA-1 cohorts 1-2. | Oluwole et al (ZUMA-1 cohort 6)28 NCT02348216 |
| Prophylactic anakinra given on days 0-7. | Adult DLBCL treated with axi-cel. | Prophy anakinra: grade ≥2 CRS, 40%. Grade ≥3 ICANS, 20% | No prophy anakinra, tumor burden–matched retrospective cohort: grade ≥2 CRS, 70%. Grade ≥3 ICANS, 50%. | Early follow-up suggests efficacy preserved. | Strati et al29 NCT04432506 |
| Prophylactic anakinra. Started at first fever, or day 2 if no fever. Continued for a minimum of 10 days. | Adult DLBCL and MCL treated with axi-cel, tisa-cel, and brexu-cel. | Prophy anakinra: grade ≥3 CRS, 6%. Grade ≥3 ICANS, 6%. | No specific comparison cohort. | Early follow-up suggests efficacy preserved. | Park et al30 NCT04148430 |
| Early intervention during low-grade CRS | |||||
| Intervention with tocilizumab and/or corticosteroids for persistent grade 1 or any grade 2 CRS/ICANS. | Adult DLBCL treated with axi-cel. | Early intervention: grade ≥3 CRS, 2%. Grade ≥3 ICANS, 17%. | No early intervention (ZUMA-1 cohorts 1-2): grade ≥3 CRS, 13%. Grade ≥3 ICANS, 28%. | Lower baseline tumor burden than ZUMA-1 cohorts 1-2. | Topp et al (ZUMA-1 cohort 4)31 NCT02348216 |
| Tocilizumab at CRS onset in patients with high tumor burdens. | Children and young adults with >40% bone marrow involvement of B-ALL treated with CD19 CAR T cells. | Early toci: grade ≥4 CRS, 27% (Penn grading scale). Grade ≥4 neurotoxicity, 7%. | No early toci prior phase 1 trial, high tumor burden: grade ≥4 CRS, 50%. Grade ≥4 neurotoxicity, 4%. | Efficacy similar to prior cohorts. | Kadauke et al3 NCT02906371 |
| Tocilizumab and/or corticosteroids if persistent CRS. | Children and young adults with B-ALL treated with CD19 CAR T cells. | Early intervention: severe CRS, 15%. Grade ≥3 neurotoxicity, 22%. | No early intervention, cohort on same trial treated in DLT phase: severe CRS, 30%. Grade ≥3 neurotoxicity, 25%. | Used a study-specific definition of severe CRS. Efficacy appeared preserved. | Gardner et al4 NCT02028455 |
| Concurrent BTK inhibition | |||||
| Ibrutinib + CAR T cells. | Adult CLL treated with CD19 CAR T cells. | Concurrent ibrutinib: grade ≥3 CRS, 0%. Grade ≥3 ICANS, 26%. | No concurrent ibrutinib, earlier cohort of same trial: grade ≥3 CRS, 11%. Grade ≥3 ICANS, NA. | Better CAR T-cell expansion with concurrent ibrutinib, no difference in efficacy. | Gauthier et al32 NCT01865617 |
| Concurrent JAK inhibition | |||||
| Itacitinib + CAR T cells. | Adult DLBCL or MCL (90% of patients) treated with axi-cel, tisa-cel, or brexu-cel. | Concurrent itacitinib: grade ≥3 CRS 2%. Grade ≥3 ICANS, 13%. | No specific comparison cohort. | Randomized phase 2 (itacitinib vs placebo) underway, treating DLBCL/FL with axi-cel. | Pratta et al33 NCT04071366 |
| Strategy . | Disease/product . | Outcome . | Comparison∗ . | Comments . | Reference . |
|---|---|---|---|---|---|
| Fractionated CAR T-cell dosing | |||||
| Fractionated dosing: day 1 (10% dose), day 2 (30%), and day 3 (60%), with day 2 and day 3 doses allowed to be held for early CRS. | Adult B-ALL treated with CD19 CAR T cells. | Fractionated dose: grade ≥4 CRS, 5% (Penn grading scale) Grade ≥3 neurotoxicity, 6%. | High fixed dose: grade ≥4 CRS, 50%; 3 of 6 patients died. | Difficult to implement with fixed-dose commercial CAR T-cell products. | Frey et al25 NCT02030847 |
| Prophylaxis | |||||
| Prophylactic tocilizumab given on day 2. | Adult DLBCL treated with axi-cel. | Prophy toci: grade ≥3 CRS, 3%. Grade ≥3 ICANS, 41%. One case of cerebral edema. | No prophy toci (ZUMA-1 cohorts 1-2)26: grade ≥3 CRS, 13%. Grade ≥3 ICANS, 28%. | Peak IL-6 levels were higher in the prophy toci group, possibly because IL-6R antagonists increase free IL-6. | Locke et al (ZUMA-1 cohort 3)27 NCT02348216 |
| Prophylactic dexamethasone 10 mg on days 0, 1, and 2. | Adult DLBCL treated with axi-cel. | Prophy dex: grade ≥3 CRS, 0%. Grade ≥3 ICANS, 13%. | No prophy dex (ZUMA-1 cohorts 1-2): grade ≥3 CRS, 13%. Grade ≥3 ICANS, 28%. | Lower baseline tumor burden than ZUMA-1 cohorts 1-2. | Oluwole et al (ZUMA-1 cohort 6)28 NCT02348216 |
| Prophylactic anakinra given on days 0-7. | Adult DLBCL treated with axi-cel. | Prophy anakinra: grade ≥2 CRS, 40%. Grade ≥3 ICANS, 20% | No prophy anakinra, tumor burden–matched retrospective cohort: grade ≥2 CRS, 70%. Grade ≥3 ICANS, 50%. | Early follow-up suggests efficacy preserved. | Strati et al29 NCT04432506 |
| Prophylactic anakinra. Started at first fever, or day 2 if no fever. Continued for a minimum of 10 days. | Adult DLBCL and MCL treated with axi-cel, tisa-cel, and brexu-cel. | Prophy anakinra: grade ≥3 CRS, 6%. Grade ≥3 ICANS, 6%. | No specific comparison cohort. | Early follow-up suggests efficacy preserved. | Park et al30 NCT04148430 |
| Early intervention during low-grade CRS | |||||
| Intervention with tocilizumab and/or corticosteroids for persistent grade 1 or any grade 2 CRS/ICANS. | Adult DLBCL treated with axi-cel. | Early intervention: grade ≥3 CRS, 2%. Grade ≥3 ICANS, 17%. | No early intervention (ZUMA-1 cohorts 1-2): grade ≥3 CRS, 13%. Grade ≥3 ICANS, 28%. | Lower baseline tumor burden than ZUMA-1 cohorts 1-2. | Topp et al (ZUMA-1 cohort 4)31 NCT02348216 |
| Tocilizumab at CRS onset in patients with high tumor burdens. | Children and young adults with >40% bone marrow involvement of B-ALL treated with CD19 CAR T cells. | Early toci: grade ≥4 CRS, 27% (Penn grading scale). Grade ≥4 neurotoxicity, 7%. | No early toci prior phase 1 trial, high tumor burden: grade ≥4 CRS, 50%. Grade ≥4 neurotoxicity, 4%. | Efficacy similar to prior cohorts. | Kadauke et al3 NCT02906371 |
| Tocilizumab and/or corticosteroids if persistent CRS. | Children and young adults with B-ALL treated with CD19 CAR T cells. | Early intervention: severe CRS, 15%. Grade ≥3 neurotoxicity, 22%. | No early intervention, cohort on same trial treated in DLT phase: severe CRS, 30%. Grade ≥3 neurotoxicity, 25%. | Used a study-specific definition of severe CRS. Efficacy appeared preserved. | Gardner et al4 NCT02028455 |
| Concurrent BTK inhibition | |||||
| Ibrutinib + CAR T cells. | Adult CLL treated with CD19 CAR T cells. | Concurrent ibrutinib: grade ≥3 CRS, 0%. Grade ≥3 ICANS, 26%. | No concurrent ibrutinib, earlier cohort of same trial: grade ≥3 CRS, 11%. Grade ≥3 ICANS, NA. | Better CAR T-cell expansion with concurrent ibrutinib, no difference in efficacy. | Gauthier et al32 NCT01865617 |
| Concurrent JAK inhibition | |||||
| Itacitinib + CAR T cells. | Adult DLBCL or MCL (90% of patients) treated with axi-cel, tisa-cel, or brexu-cel. | Concurrent itacitinib: grade ≥3 CRS 2%. Grade ≥3 ICANS, 13%. | No specific comparison cohort. | Randomized phase 2 (itacitinib vs placebo) underway, treating DLBCL/FL with axi-cel. | Pratta et al33 NCT04071366 |
axi-cel, axicabtagene ciloleucel; brexu-cel, brexucabtagene autoleucel; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; DLT, dose-limiting toxicity; FL, follicular lymphoma; MCL, mantle cell lymphoma; NA, not available; prophy, prophylactic; toci, tocilizumab; tisa-cel, tisagenlecleucel.
None of the comparisons are randomized and instead provide information as compared with nonrandomized cohorts, cross-trial comparisons, and retrospective cohorts.
Case 2 (continued)
Subsequently, on day 8, despite concurrent improvement in CRS to grade 1, the patient showed global aphasia and epileptiform activity upon an electroencephalogram, consistent with grade 3 ICANS. For treatment of ICANS, she received methylprednisolone 1 gm IV daily for 2 days followed by a dexamethasone taper to discontinuation over 2 weeks. She also received anakinra 100 mg IV every 6 hours, which was tapered over a 10-day course. She had been on seizure prophylaxis with levetiracetam (500 mg daily) before CAR T-cell infusion, yet per the guidance of a neurology consultation, levetiracetam was increased to therapeutic doses and 2 additional antiepileptics, lacosamide and phenytoin, were initiated to control seizure activity. Given the need for more frequent monitoring of her neurological status, she was treated in the ICU for several days. Upon resolution of CRS and ICANS, she was noted to have CMV viremia, cytopenias, and severe deconditioning, which substantially prolonged her inpatient stay. Her day 30 positron emission tomography/CT scan showed a complete metabolic remission, and she was ultimately discharged from the hospital on day 65 after CAR T-cell infusion.
As noted in our cases, corticosteroids are generally accepted for refractory CRS (as in case 1) and as first-line therapy for ICANS (as in case 2) and are prescribed for treatment of concurrent CRS and ICANS (also noted in case 2).38 Dexamethasone is frequently recommended for ICANS because it crosses the blood-brain barrier.10 However, package inserts for US Food and Drug Administration (FDA)-approved CD19 CAR T-cell products recommend dexamethasone or methylprednisolone for the treatment of severe ICANS.15-17
There are conflicting data on the impact that corticosteroid exposure has on CAR T-cell efficacy, with some studies noting no change in the efficacy,4,8,28 whereas others show decreased efficacy.7,39 Nonetheless, for recurrent, concurrent, or refractory toxicities, patients often receive prolonged courses of high-dose corticosteroids. Although corticosteroids aim to decrease CAR T-cell–associated inflammation, they also cause several deleterious side effects, including hyperglycemia, muscle weakness, and increased risk of infection. Accordingly, it is often not necessary for patients to have complete resolution of CRS/ICANS before weaning or discontinuing corticosteroids, and time alone may aid in resolution of symptoms because the inflammatory state is generally self-limited.
Given the data in support of the role of IL-1 in the pathophysiology of ICANS, including elevations in the cerebrospinal fluid associated with ICANS40 and mechanistic preclinical models of CAR T-cell–associated toxicities elucidating the impact of IL-1 and improvement after its blockade,41,42 anakinra has been increasingly used to treat refractory ICANS. Anakinra is a recombinant IL-1 receptor antagonist that is FDA-approved for the treatment of rheumatoid arthritis. Preliminary data from an ongoing phase 2 study testing prophylactic anakinra to prevent CRS and ICANS show promising results30; however, its evaluation among pediatric patients is urgently needed.43 The use of intrathecal corticosteroids has also been reported for use in severe or refractory ICANS, yet its use as a systemic steroid-sparing therapy warrants prospective study.44 Furthermore, the use of intrathecal therapy may be limited by concurrent thrombocytopenia and coagulopathy observed in patients with concurrent CRS.
Case 3
A 15-year-old male with constitutional trisomy 21 and relapsed B-cell acute lymphoblastic leukemia (B-ALL) was referred for commercial tisagenlecleucel. He presented with 30% bone marrow blasts with low-level circulating peripheral blasts.
Compared with the general public, patients with Down syndrome (DS) are at a higher risk of developing hematologic malignancies,45 and despite generally good outcomes,46 they are uniquely sensitive to chemotherapy-associated toxicities and do poorly with highly-intensive cytotoxic regimens, particularly in relapse. Emerging data on the use of CD19 CAR T cells in children and young adults with DS show promising response rates and safety profiles, including the incidence and severity of ICANS. These data are comparable with the data of those without DS,47 despite an increased risk of seizures when using blinatumomab in patients with DS.48 For this patient, high disease burden increased the risk of developing severe CRS.
Case 3 (continued)
The patient remained with 30% marrow involvement after bridging chemotherapy and proceeded to LD chemotherapy and CAR T-cell infusion. He was on physiologic hydrocortisone for adrenal insufficiency. He developed fever, representing grade 1 CRS, on day 5. Although initially responsive to supportive measures (eg, acetaminophen and stress-dose hydrocortisone), he developed more persistent fevers, hypotension, rising creatinine, and decreasing urine output (grade 2 CRS) over 3 days, and he was given tocilizumab 8 mg/kg for persistent grade 2 CRS to prevent grade 3 CRS.
For patients with established or suspected adrenal insufficiency (eg, from prior corticosteroid use), maintenance of physiologic dosing with escalation to stress-dosing during CRS should be considered, particularly because it may help maintain hemodynamic stability and offset the need for higher doses of corticosteroids.
Consistent with the preemptive strategies tested by Gardner et al, which applied an early intervention strategy of tocilizumab and/or corticosteroids for CRS mitigation in children and young adults with B-ALL, tocilizumab was given for persistent symptoms of mild CRS and before the development of life-threatening toxicities (eg, grade 3 CRS).4 The approach of preventing more severe CRS/ICANS in children and young adults (as opposed to treating grade 1 CRS in older more frail adults) may be a unique consideration of younger patients in which baseline comorbidities may be less severe. Hence, patients may be able to better tolerate grade 2 CRS/ICANS without more severe complications. In patients with DS, unique comorbidities (eg, tolerance of fluid shifts in the setting of congenital cardiac complications) may necessitate consideration of earlier interventions.
Case 3 (continued)
After tocilizumab, the patient became afebrile, and CRS resolved. Approximately 10 days later, he developed a rapidly rising ferritin level (>100 000 μg/L) and hepatic transaminitis (>10 × upper limit of normal [ULN]), disproportional to C-reactive protein, which was decreasing. The patient also developed severe hypofibrinogenemia (<100 mg/dL), although prothrombin time and partial thromboplastin time were normal. Despite stable vital signs, laboratory parameters worsened, prompting the initiation of methylprednisolone (2 mg/kg per day) and anakinra (8 mg/kg per day) to treat hemophagocytic lymphohistiocytosis (HLH)-like complications. After a short course of steroids and 1 week of anakinra with tapering doses, laboratory values normalized. He achieved a minimal residual disease–negative complete response. Given additional immunosuppression, he remained on antifungal prophylaxis while on both high-dose steroids and anakinra.
HLH-like toxicities are increasingly being recognized as a potential complication of CAR T-cell–based therapies and can be severe and life-threatening. Recently termed as immune effector cell–associated HLH-like syndrome (IEC-HS) through an American Society for Transplantation and Cellular Therapy (ASTCT) effort, this hyperinflammatory syndrome manifests with cytopenia, hepatic dysfunction, hypofibrinogenemia, and hyperferritinemia.49 Because both primary/familial and secondary forms of HLH are pathologically linked to T-cell activation and immune dysregulation, this complication can be particularly challenging to diagnose with underlying hematologic malignancies.50 Initially described as part of severe CRS,51-53 more recent reports have described delayed manifestations of HLH-like toxicities even with low-grade CRS, particularly in patients receiving CD22 CAR T cells.54-61 In B-cell maturation antigen (BCMA)-targeting CAR T-cell therapies, in which severe CRS is less frequent, HLH-or macrophage activation syndrome-like toxicities are also becoming more apparent54 and are listed as potential toxicities on the FDA-approved package inserts.
In case 3, anakinra and corticosteroids were used as first-line strategies based on the established role of both agents in treatment of primary62 or secondary HLH.63,64 This approach has been recently endorsed by the ASTCT Committee on Cellular Therapy, although further study is needed.49 Importantly, given the clinical presentation and stable vital signs (eg, afebrile and normotensive), tocilizumab was not indicated as per standard CRS guidelines, highlighting the need to distinguish the presentation and treatment of IEC-HS. Multicenter efforts to establish the clear criteria to facilitate an improved understanding of this toxicity and formulate the optimal treatment approach are ongoing. This is particularly important in cases of refractory IEC-HS that are nonresponsive or progressive to first-line approaches. Therapeutic strategies for refractory IEC-HS remain under study but include ruxolitinib,65 emapalumab,66 and etoposide.49
Prevention of severe and/or refractory CRS/ICANS
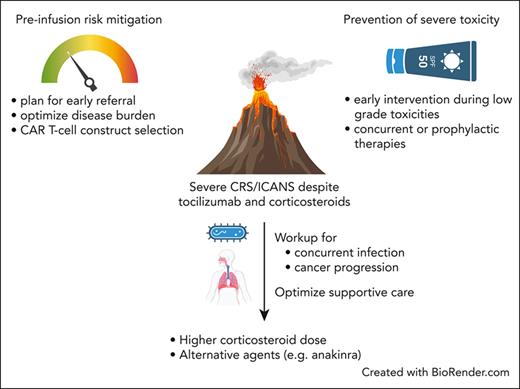
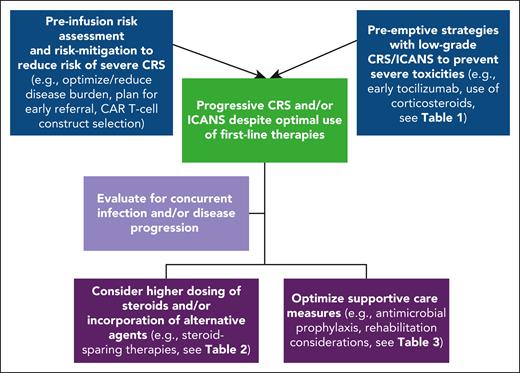
Managing refractory CRS and ICANS after it has already developed remains a challenge. Importantly, because not all severe cases are refractory and may be effectively managed with tocilizumab and corticosteroids alone, distinguishing toxicities responding to first-line approaches from those in which additional investigations and/or interventions are needed is a critical consideration. Nonetheless, because developing severe or refractory toxicities impairs outcomes, early identification and pre-emptive interventions in those at high risk may help improve outcomes. Several principles emerging from clinical trials and real-world settings provide insights into risk factors associated with severe CRS. Risk-mitigation preventive strategies for those at high risk of toxicity may improve outcomes (Table 1; Figure 1).
Approach to mitigate severe CRS/ICANS and progressive toxicities despite the optimal use of first-line therapies.
Approach to mitigate severe CRS/ICANS and progressive toxicities despite the optimal use of first-line therapies.
Disease type and biology
The underlying cancer diagnosis influences toxicity, with highly proliferative aggressive diseases, such as DLBCL and B-ALL, at higher risk of severe CRS/ICANS than diseases, such as follicular lymphoma or myeloma.67 Similarly, within indolent lymphomas, marginal zone lymphoma has a higher rate of CRS/ICANS than follicular lymphoma,68 and transformed marginal zone lymphoma may be at higher risk rate than transformed follicular lymphoma or de novo DLBCL. However, severe and refractory CRS and ICANS may occur in any disease type, especially among patients who are heavily pretreated and refractory. For example, a recent analysis of myeloma patients treated with BCMA CAR T-cell therapy in the real-world setting reported 3 deaths attributed to CRS or neurotoxicity.69 At present, management recommendations for cases of severe and refractory CRS or ICANS are generalizable across disease types. Finally, emerging evidence suggests that tumor biology affects the risk of toxicity, given that the increased regulatory T cells in the tumor microenvironment has been linked to decreased risk of ICANS in patients with DLBCL.70,71
Disease burden
A high tumor burden is a key risk factor for toxicity, often associated with higher inflammatory cytokine levels and CAR T-cell expansion.12,72-76 High disease burden is also associated with an increased risk of relapse after CAR T-cell therapy even though remission rates are often comparable.75,77-79 Furthermore, patients who experience severe CRS are at a higher risk for CAR T-cell failure.80 Accordingly, patients with rapidly progressive disease, high tumor burdens, or poor performance status may be better candidates for therapies other than CAR T cells. Similarly, systems issues such as late referrals, delays in insurance approval, or difficulty in manufacturing the CAR product may increase patient risks as the tumor burden grows and performance status worsens.81 In some instances, debulking patients before CAR T-cell therapy can be tried, although patients receive CAR T-cell therapy because they are resistant to other treatments. Therefore, patients should be identified early and moved toward CAR T-cell infusion as quickly as possible. For large masses, debulking radiation is often used as bridging therapy; however, radiation can be inflammatory and may not lower CRS risk, and the optimal timing, dose, and field for bridging radiation therapy are unknown.82-84
CAR T-cell construct
CAR T-cell products vary in the likelihood of causing severe toxicity and depending on the disease treated. For instance, in DLBCL with CD19 CAR T cells, CD28-costimulated CAR T-cell products demonstrate higher rates of CRS and ICANS compared with 4-1BB-costimulated products.13 In addition, ICANS may be more severe when targeting CD19, potentially because of the on-target, off-tumor targeting of CD19 on brain mural cells.85 Unique movement-related toxicities have been observed when targeting BCMA, possibly because of off-tumor expression of these targets in, and around, the brain (discussed in more detail in an accompanying “How I Treat” article122).85,86 Even when the target and costimulatory domain are identical, kinetics of toxicity may differ between products, which, in turn, affect clinical management. With myeloma, CRS associated with idecabtagene vicleucel typically starts within a few days, whereas with ciltacabtagene autoleucel, CRS may start as late as a week after infusion. When multiple CAR T-cell products are available for a given disease, consideration of a product with potentially lower toxicity should be balanced against any perceived efficacy differences between products, because tumor eradication is the goal. Development of novel CAR T-cell constructs designed to reduce toxicity are actively being developed.
Despite best efforts, patients may remain at high risk at CAR T-cell infusion. Although data are limited, the prophylaxis and early treatment of CRS appear to play a role in preventing subsequent severe toxicity (Table 1). Interpreting these single-arm trials can be challenging because rates of severe CRS and ICANS depend on histology, tumor burden, and CAR T-cell product type. Caution is needed because there are limited data on the effect of these interventions on antitumor efficacy, and patients at lower risk typically do not need these interventions. Greater attention to supportive care is also warranted, because patients with high tumor burden and inflammatory markers are also at a higher risk of developing cytopenias and infections.19,87 In addition, because conventional treatment paradigms and data on the impact of interventions on CAR T-cell efficacy are derived from experiences with B-cell malignancies, increased vigilance will be needed to optimize treatment of new emergent toxicities across novel constructs or diseases, in which unique considerations may necessitate alternative approaches.
Recent efforts to identify biomarkers or develop risk scores (eg, CAR-HEMATOTOX and mEASIX) based on clinical laboratory parameters remain of great interest in aiding further risk stratification for associations with inflammation, delayed toxicities, or overall outcomes.19,87-90 Further study of such biomarkers is warranted to determine the ability to predict poor response to standard CRS/ICANS interventions.
Pharmacologic interventions for severe and/or refractory toxicities
Evidence-based data on the use of pharmacologic interventions for treatment of severe and/or refractory toxicities (Table 2) are generally lacking and limited to single-institutional experiences or case reports, preclinical data, T-cell–directed mechanisms of action, or adopted for use based on efficacy in alternative hyperinflammatory settings. Risk of infection with additive use of immunosuppressive agents is a concern and consultation with infectious disease specialists is suggested to optimize infection surveillance strategies and use of prophylactic/preemptive antimicrobials.22
Pharmacologic strategies being explored for potential use in severe/refractory CRS/ICANS
| Therapeutic category . | Agent . | Mechanism of action . | FDA-approved indication . | Description of use with CRS/ICANS . | Prospective study in CRS/ICANS . |
|---|---|---|---|---|---|
| Anti-cytokine directed | Anakinra | IL-1 receptor antagonist | Reduction in signs/symptoms and to slow the progression of damage in adults with moderately to severely active RA who have failed ≥1 DMARDs, or for treatment of neonatal-onset multisystem inflammatory disorder. | Preclinical data supporting the role of IL-1 in mediating CRS/ICANS, alongside the impact of IL-1 blockade in treatment of CAR T-cell toxicities.41,42 Other clinical experience has been largely based on single-center/limited patient experiences and/or anecdotal reports. Has been used for treatment of refractory CRS/ICANs and HLH-like toxicities.20,43,55-57,91-95 Prospective studies are ongoing.30 Can be administered by IV or SC, with preference for IV administration in patients with edema in whom SC administration may not be as reliable. Given the short half-life with IV administration, more frequent dosing may be required.11 | NCT04148430 NCT04359784 NCT04150913 |
| Siltuximab | IL-6 antagonist | In adults, for the treatment of patients with multicentric Castleman disease who are HIV- and HHV-8–negative. | Mostly, the use has been limited to second-line or refractory CRS/ICANS after the use of multiple other agents. Limited data available.96,97 | NCT04975555 | |
| Emapalumab | IFN-γ–blocking antibody | For the treatment of adult and pediatric patients with primary HLH with refractory, recurrent, or progressive disease or intolerance to conventional HLH therapy. | Preclinical data supporting the role of IFN-γ in mediating CRS/ICANS, alongside the impact of IFN-γ blockade in treatment of CAR T-cell toxicities.98 Clinical experience is limited.66 | ||
| T-cell targeted | Antithymocyte globulin (ATG) | Direct T-cell targeting | For prophylaxis and treatment of acute rejection in patients receiving a kidney transplant, or use in conjunction with concomitant immunosuppression. | Potential use is based on clinical efficacy of targeting T cells. Data on CRS/ICANS are limited.99 Risk of infection/immunosuppression is high. | |
| Alemtuzumab (anti-CD52) | Depletion of T and B cells by binding to CD52 on the cell surface | For treatment of patients with relapsing forms of MS. | No published reports on its use for treatment of relapsed/refractory CRS/ICANS. Emerging use to facilitate engraftment of allogeneic or off-the-shelf CAR T cells.100,101 | ||
| Cyclophosphamide | Alkylating agent targeting T cells | Multiple indications for use in pediatrics and adults with malignancies and minimal change nephrotic syndrome. | Could be used for eradicating T cells. Limited experience (single case report) in the use for refractory CRS/ICANS.102 | ||
| TKIs103 | Dasatinib | TKI (BCR-ABL) | For adults in chronic, accelerated, or blast phase of Ph+ CML; for adults with Ph+ ALL. | Preclinical studies demonstrate the ability of dasatinib to suppress CAR T-cell cytotoxicity, cytokine secretion, and proliferation.104,105 | NCT04603872 |
| Ibrutinib | BTK inhibitor | For adults with MCL who have received at least 1 prior therapy, for CLL with 17p deletion, or in those who have received at least 1 prior therapy or who have Waldenstrom macroglobulinemia. | Based on the role of ibrutinib to inhibit IL-2–induced tyrosine kinases, there is evidence of reduction in cytokine production in a preclinical model of CD19 CAR T cells.106 Emerging clinical data incorporating ibrutinib suggest the potential of reducing CRS severity.32 | NCT03960840 | |
| Ruxolitinib or alternative JAK1 inhibitors | JAK inhibitor | For treatment of adults with myelofibrosis and polycythemia vera. For treatment of adults and pediatric patients aged >12 y with steroid refractory acute GVHD or chronic GVHD after failure of >1-2 lines of systemic therapy. | Preclinical studies demonstrate a role of JAK pathway singling blockade and dose-dependent reduction of multiple cytokines implicated in CRS.107 Patient experience for use in CRS/ICANS is limited to case reports.108,109 | ||
| CAR T-cell construct–based safety switches | Based on the CAR T-cell construct and the incorporation of either suicide switches (eg, inducible Caspase 9 targeted by the synthetic dimerizing drug rimiducid)110 alternative transcriptional controls,111 or truncated-targetable receptors (eg, EGFR or CD20) that can be targeted by monoclonal antibodies (eg, cetuximab or rituximab), these agents can be considered for use when eradicating the CAR T cell in the setting of refractory and when life-threatening CAR T-cell–mediated toxicities are present. The clinical use and experience to date are limited. | ||||
| Therapeutic category . | Agent . | Mechanism of action . | FDA-approved indication . | Description of use with CRS/ICANS . | Prospective study in CRS/ICANS . |
|---|---|---|---|---|---|
| Anti-cytokine directed | Anakinra | IL-1 receptor antagonist | Reduction in signs/symptoms and to slow the progression of damage in adults with moderately to severely active RA who have failed ≥1 DMARDs, or for treatment of neonatal-onset multisystem inflammatory disorder. | Preclinical data supporting the role of IL-1 in mediating CRS/ICANS, alongside the impact of IL-1 blockade in treatment of CAR T-cell toxicities.41,42 Other clinical experience has been largely based on single-center/limited patient experiences and/or anecdotal reports. Has been used for treatment of refractory CRS/ICANs and HLH-like toxicities.20,43,55-57,91-95 Prospective studies are ongoing.30 Can be administered by IV or SC, with preference for IV administration in patients with edema in whom SC administration may not be as reliable. Given the short half-life with IV administration, more frequent dosing may be required.11 | NCT04148430 NCT04359784 NCT04150913 |
| Siltuximab | IL-6 antagonist | In adults, for the treatment of patients with multicentric Castleman disease who are HIV- and HHV-8–negative. | Mostly, the use has been limited to second-line or refractory CRS/ICANS after the use of multiple other agents. Limited data available.96,97 | NCT04975555 | |
| Emapalumab | IFN-γ–blocking antibody | For the treatment of adult and pediatric patients with primary HLH with refractory, recurrent, or progressive disease or intolerance to conventional HLH therapy. | Preclinical data supporting the role of IFN-γ in mediating CRS/ICANS, alongside the impact of IFN-γ blockade in treatment of CAR T-cell toxicities.98 Clinical experience is limited.66 | ||
| T-cell targeted | Antithymocyte globulin (ATG) | Direct T-cell targeting | For prophylaxis and treatment of acute rejection in patients receiving a kidney transplant, or use in conjunction with concomitant immunosuppression. | Potential use is based on clinical efficacy of targeting T cells. Data on CRS/ICANS are limited.99 Risk of infection/immunosuppression is high. | |
| Alemtuzumab (anti-CD52) | Depletion of T and B cells by binding to CD52 on the cell surface | For treatment of patients with relapsing forms of MS. | No published reports on its use for treatment of relapsed/refractory CRS/ICANS. Emerging use to facilitate engraftment of allogeneic or off-the-shelf CAR T cells.100,101 | ||
| Cyclophosphamide | Alkylating agent targeting T cells | Multiple indications for use in pediatrics and adults with malignancies and minimal change nephrotic syndrome. | Could be used for eradicating T cells. Limited experience (single case report) in the use for refractory CRS/ICANS.102 | ||
| TKIs103 | Dasatinib | TKI (BCR-ABL) | For adults in chronic, accelerated, or blast phase of Ph+ CML; for adults with Ph+ ALL. | Preclinical studies demonstrate the ability of dasatinib to suppress CAR T-cell cytotoxicity, cytokine secretion, and proliferation.104,105 | NCT04603872 |
| Ibrutinib | BTK inhibitor | For adults with MCL who have received at least 1 prior therapy, for CLL with 17p deletion, or in those who have received at least 1 prior therapy or who have Waldenstrom macroglobulinemia. | Based on the role of ibrutinib to inhibit IL-2–induced tyrosine kinases, there is evidence of reduction in cytokine production in a preclinical model of CD19 CAR T cells.106 Emerging clinical data incorporating ibrutinib suggest the potential of reducing CRS severity.32 | NCT03960840 | |
| Ruxolitinib or alternative JAK1 inhibitors | JAK inhibitor | For treatment of adults with myelofibrosis and polycythemia vera. For treatment of adults and pediatric patients aged >12 y with steroid refractory acute GVHD or chronic GVHD after failure of >1-2 lines of systemic therapy. | Preclinical studies demonstrate a role of JAK pathway singling blockade and dose-dependent reduction of multiple cytokines implicated in CRS.107 Patient experience for use in CRS/ICANS is limited to case reports.108,109 | ||
| CAR T-cell construct–based safety switches | Based on the CAR T-cell construct and the incorporation of either suicide switches (eg, inducible Caspase 9 targeted by the synthetic dimerizing drug rimiducid)110 alternative transcriptional controls,111 or truncated-targetable receptors (eg, EGFR or CD20) that can be targeted by monoclonal antibodies (eg, cetuximab or rituximab), these agents can be considered for use when eradicating the CAR T cell in the setting of refractory and when life-threatening CAR T-cell–mediated toxicities are present. The clinical use and experience to date are limited. | ||||
Given the concern for increasing risk of infection with incorporation of additional agents, caution is advised against the simultaneous administration of multiple strategies.
There are currently no evidence-based guidelines or proven strategies that exist for the treatment of CRS/ICANS that is persistent or progressive after intervention with tocilizumab and corticosteroids. The table represents a list of potential agents that have been considered based on single-institutional or limited patient experiences, preclinical data, established T-cell–directed mechanism of action, or adopted for use based on efficacy in alternative hyperinflammatory settings. FDA-approved package inserts for several commercial CAR T-cell constructs advise the use of implementing alternative strategies with grade 4 CRS (eg, anakinra, siltuximab, ruxolitinib, cyclophosphamide, IVIG, and ATG).
ALL, acute lymphoblastic leukemia; BCR, B-cell receptor; BTK, Bruton tyrosine kinase; CML, chronic myelogenous leukemia; DMARDs, disease-modifying antirheumatic drugs; EGFR, epidermal growth factor receptor; GVHD, graft-versus-host disease; IFN-γ, interferon gamma; JAK, Janus kinase; MCL, mantle cell lymphoma; MS, multiple sclerosis; RA, rheumatoid arthritis; TKI, tyrosine kinase inhibitor.
Anakinra
Across all 3 refractory cases presented in this article, anakinra emerged as a common agent used after tocilizumab and/or with corticosteroids. Based on the data supporting IL-1 signaling in the pathogenesis of ICANS41,42 and its ability to cross the blood-brain barrier, it has been proposed for steroid-refractory CRS/ICANS. In early reports of patients with refractory CRS, anakinra has been reported to decrease inflammatory cytokine levels and provide some clinical responses, but variability in clinical use makes it difficult to determine the extent of benefit.20,43,44,57 As anakinra is commonly used after corticosteroids and tocilizumab, the increased immunosuppression raises concern for increased risk of infections; therefore, antimicrobial prophylaxis with fluoroquinolones and mold-directed antifungals are often initiated or intensified for patients who are not already on these agents. Prospective trials to evaluate anakinra for prevention and treatment of CRS and/or ICANS (NCT04148430, NCT04359784, and NCT04150913) will provide much needed insight into the role of this important therapeutic. In the setting of IEC-HS, anakinra alone, or in conjunction with corticosteroids, may be able to effectively mitigate this toxicity to full resolution.55,91
Anti-CAR T-cell–directed strategies
When patients have severe or refractory CRS and/or ICANS, the last resort is often to try to target the remaining CAR T cells. Anti-CAR T-cell–directed interventions may include chemotherapy, T-cell–directed therapies, tyrosine kinase inhibitors, or CAR construct–directed therapies (Table 2). However, experience is limited, and infection risk of such strategies may be particularly high, especially when given after multiple prior immunosuppressive approaches.
Supportive care
When patients develop high-grade, recurrent, or refractory CAR-mediated toxicity, they are at a risk of increased morbidity and mortality.112 Often, management of CRS and/or ICANS results in a protracted hospital stay, increased exposure to immunosuppressive medications, greater risk of infection, and worsening of the patient’s functional status. To diminish the impact that these unintended consequences may have on the long-term benefit of CAR T cells, attention should be given to supportive care measures (Table 3).
Supportive care measures for patients with severe/refractory CRS or ICANS
| Neurology |
| • Identify patients at high risk of developing ICANS and engage neurology early113 |
| • Tailor duration of steroids in patients with severe ICANS to treat toxicity but limit adverse side effects |
| Immune/hematologic |
| • Limit the duration of high-dose steroids for the management of toxicity to diminish immune suppression |
| • Use cytokine-directed treatments, such as anakinra, as a steroid-sparing approach20,43,57 |
| • Optimize approaches to treat ongoing cytopenias |
| Infectious disease |
| • Use prophylactic antimicrobials, such as trimethoprim-sulfamethoxazole, for Pneumocystis jirovecii prophylaxis22 |
| • Use prophylactic antivirals, such as acyclovir or valacyclovir, for herpes virus prophylaxis before conditioning chemotherapy22 |
| • Practice antibiotic stewardship with broad-spectrum antibiotics and blood cultures for patients with neutropenic fever114,115 |
| • Assess for CMV, adeno- or other viremias in patients with persistent cytopenia after treatment |
| • Optimize antifungal prophylaxis in patients with prolonged immunosuppression due to CRS/ICANS management and cytopenias |
| Rehabilitation |
| • Involve inpatient rehabilitation services for patients, particularly those with lengthy hospital stays or those who received prolonged steroids, after they are clinically stable |
| • Encourage engagement of caregivers and social workers |
| Cognitive/psychosocial |
| • Consult psychiatry or psychiatric oncology to aid in the management of delirium, particularly in older or pediatric patients who develop ICANS |
| Neurology |
| • Identify patients at high risk of developing ICANS and engage neurology early113 |
| • Tailor duration of steroids in patients with severe ICANS to treat toxicity but limit adverse side effects |
| Immune/hematologic |
| • Limit the duration of high-dose steroids for the management of toxicity to diminish immune suppression |
| • Use cytokine-directed treatments, such as anakinra, as a steroid-sparing approach20,43,57 |
| • Optimize approaches to treat ongoing cytopenias |
| Infectious disease |
| • Use prophylactic antimicrobials, such as trimethoprim-sulfamethoxazole, for Pneumocystis jirovecii prophylaxis22 |
| • Use prophylactic antivirals, such as acyclovir or valacyclovir, for herpes virus prophylaxis before conditioning chemotherapy22 |
| • Practice antibiotic stewardship with broad-spectrum antibiotics and blood cultures for patients with neutropenic fever114,115 |
| • Assess for CMV, adeno- or other viremias in patients with persistent cytopenia after treatment |
| • Optimize antifungal prophylaxis in patients with prolonged immunosuppression due to CRS/ICANS management and cytopenias |
| Rehabilitation |
| • Involve inpatient rehabilitation services for patients, particularly those with lengthy hospital stays or those who received prolonged steroids, after they are clinically stable |
| • Encourage engagement of caregivers and social workers |
| Cognitive/psychosocial |
| • Consult psychiatry or psychiatric oncology to aid in the management of delirium, particularly in older or pediatric patients who develop ICANS |
Medical
As discussed earlier, patients with severe or refractory CRS and/or ICANS typically receive prolonged courses of high-dose corticosteroids with other immunosuppressive agents. The recommendations delineated in Table 3 primarily focus on strategies to limit the adverse effects of these medications. First, to mitigate the deleterious effects of corticosteroids, the use of steroid-sparing, cytokine-directed medications for prophylaxis, or treatment of CAR-mediated toxicity should be assessed.6
Second, several studies have found that infectious complications are frequent after CAR T-cell therapy. Severe CRS and prolonged corticosteroid exposure are risk factors for severe infections.19,116-119 Given these findings, prophylactic antimicrobials are essential to reduce infections, including emergence of latent and opportunistic infections.115 Although exposure to broad-spectrum antibiotics targeting obligate anaerobes (eg, piperacillin/tazobactam, meropenem, and imipenem/cilastatin) within the 4 weeks before CAR T-cell therapy has been associated with worse outcomes, possibly because of alterations in the intestinal microbiome,114 the impact of antibiotics administered after CAR T-cell therapy is not yet known.
Rehabilitation and cognitive and psychosocial considerations
Multiple factors, including CRS and ICANS, immobility, malnutrition, infection, and the catabolic effects of prolonged corticosteroids (Table 2), may lead to a deterioration in the conditioning and mental status of patients after CAR T-cell therapy. For such patients, early considerations for rehabilitation to address deconditioning and steroid myopathy that may result in proximal limb weakness is needed.120 Emerging research also suggests the presence of short- and long-term biobehavioral effects of CAR T-cell therapy,121 but more work is needed in this area to understand the long-term implications for patients who experience severe or refractory CAR-mediated toxicities.
Conclusions
Given the tremendous potential of CAR T cells, preventing and effectively managing severe toxicities remain the highest priority. Although we await results from prospective trials testing novel approaches, we outline considerations that are critical to the optimal management of those experiencing refractory toxicities.
Acknowledgments
The authors thank Anna Nikonova and Aliyah Baluch for their assistance with the cases. The authors acknowledge the efforts of the American Society of Hematology’s Subcommittee on Emerging Gene and Cell Therapies for their support in prioritizing the need to discuss emergent CAR T-cell toxicities.
This work was supported, in part, by the Intramural Research Program, National Cancer Institute and NIH Clinical Center, National Institutes of Health (ZIA BC 011823) (N.N.S). M.S. is supported by the following funding sources: Burroughs Wellcome Fund, Damon Runyon Cancer Research Foundation, V Foundation, Emerson Collective, American Cancer Society, American Society of Hematology-Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program, and the National Institutes of Health (NHLBI K08 HL156082-01A1). M.D.J. is supported by the Mark Foundation, the Florida Academic Cancer Center Alliance, and the Bankhead-Coley Cancer Research Program.
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authorship
Contribution: M.D.J., M.S., and N.N.S. contributed to the writing of the manuscript and agreed to the contents of the manuscript before submission.
Conflict-of-interest disclosure: M.D.J. declares a consultancy/advisory role for Kite/Gilead, BMS, Novartis, and MyeloidTx and research funding from Kite/Gilead and Incyte. N.N.S. receives royalties from CARGO Therapeutics and has participated in advisory boards for Sobi and VOR. M.S. declares a consultancy/advisory role for BMS.
Correspondence: Nirali N. Shah, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Building 10, 1W-5750, Bethesda, MD 20892; e-mail: nirali.shah@nih.gov.
References
Author notes
∗M.D.J. and M.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal