Abstract
Increasing use of chimeric antigen receptor T-cell therapy (CAR-T) has unveiled diverse toxicities warranting specific recognition and management. Cytopenias occurring after CAR-T infusion invariably manifest early (<30 days), commonly are prolonged (30-90 days), and sometimes persist or occur late (>90 days). Variable etiologies of these cytopenias, some of which remain incompletely understood, create clinical conundrums and uncertainties about optimal management strategies. These cytopenias may cause additional sequelae, decreased quality of life, and increased resource use. Early cytopenias are typically attributed to lymphodepletion chemotherapy, however, infections and hyperinflammatory response such as immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome may occur. Early and prolonged cytopenias often correlate with severity of cytokine release syndrome or immune effector cell–associated neurotoxicity syndrome. Bone marrow biopsy in patients with prolonged or late cytopenias is important to evaluate for primary disease and secondary marrow neoplasm in both pediatric and adult patients. Commonly, cytopenias resolve over time and evidence for effective interventions is often anecdotal. Treatment strategies, which are limited and require tailoring based upon likely underlying etiology, include growth factors, thrombopoietin-receptor agonist, stem cell boost, transfusion support, and abrogation of infection risk. Here we provide our approach, including workup and management strategies, for cytopenias after CAR-T.
Introduction
Chimeric antigen receptor T-cell therapy (CAR-T) has brought a paradigm shift in the treatment of B-cell lymphoma (BCL), B-cell acute lymphoblastic leukemia (B-ALL), and multiple myeloma (MM).1,2 Numerous trials are testing CAR-Ts against different targets as treatment for other malignant and nonmalignant conditions. Cytopenia in ≥1 cell lineages often occurs after CAR-T and may cause additional sequelae, decreased quality of life, and increased use of health care resources.3-6 There are several underlying pathophysiological mechanisms that may cause these cytopenias and can be classified to some degree by considering the timeline from CAR-T infusion.7,8 Cytopenias invariably manifest early (<30 days after infusion), commonly may be prolonged (30-90 days after infusion), and sometimes persist or occur late (>90 days after infusion). Although lymphodepletion (LD) chemotherapy contributes to early cytopenias, prolonged cytopenias can be multifactorial and seem to be closely associated with CAR-T and disease-associated inflammation. Similarly, late cytopenias are likely unrelated to the LD and additional underlying etiologies should be explored, including therapy-related myeloid neoplasms, disease relapse, infection, and ongoing immune disturbances.
Cytopenias increase morbidity and mortality after CAR-T. Infection risk associated with leukopenia is compounded by longstanding hypogammaglobulinemia after CD19- and B-cell maturation antigen (BCMA)-targeting CAR-T, increasing the incidences of severe viral, bacterial, and fungal infections.7-10 Persistent thrombocytopenia increases the risk of bleeding complications, particularly within the first 30 days.11 Platelet and packed red blood cell (PRBC) transfusion support may be necessary and disrupt the quality of life. Although not systematically evaluated, many patients receive growth factor support, further increasing the resource use. Here we review our workup and management approach to cytopenias commonly encountered after CAR-T, with special attention to their severity and when they occur.
Case 1
A 65-year-old man presented with relapsed stage 4B high-grade BCL, with bulky abdominal disease and a left nasopharyngeal mass, status post rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and rituximab plus ifosfamide, carboplatin, etoposide followed by consolidative autologous transplant 4 months prior. Before LD, he had normocellular marrow without lymphomatous disease and moderately decreased absolute neutrophil count (ANC) of 990/mm3, hemoglobin 9.5 g/dL, and platelets 125 × 103/μL, attributed to bridging chemotherapy given after leukapheresis. He was treated with fludarabine and cyclophosphamide LD followed by axicabtagene ciloleucel (axi-cel), complicated by cytokine release syndrome (CRS) from day +2 to +3 (maximum grade 2), and immune effector cell–associated neurotoxicity syndrome (ICANS) from day +5 to +10 (maximum grade 3), treated with 2 doses of tocilizumab on day +3 and dexamethasone until day +10. ANC nadir was 20/mm3 on day +2, followed by a recovery to >500/mm3 on day 15. Over the course of the next 3 weeks, ANC remained under 1000/mm3 and he required PRBC and platelet transfusions every 3 to 4 days to maintain a goal of hemoglobin >7 g/dL and platelets >10 × 103/μL.
Early (<30 days from infusion) cytopenias
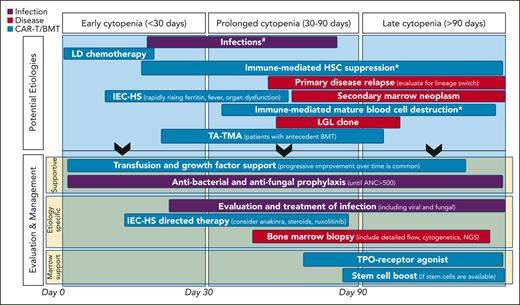
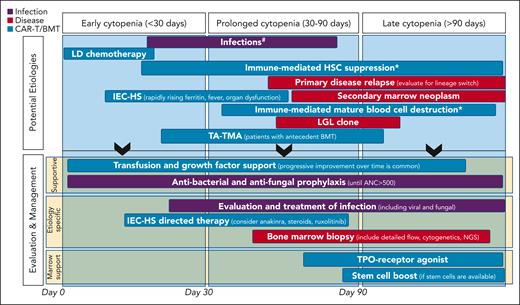
Cytopenias are commonly reported after both CD19- and BCMA-directed CAR-T (Table 1).12-22 These are typically severe, with grade 3 to 4 being the most common in the neutrophil lineage.4,5 Variable mechanisms contribute to cytopenias after CAR-T, some of which remain incompletely understood. Attention to when the cytopenia occurs in relation to the CAR-T infusion can help tailor the differential diagnosis of the cause and inform workup and treatment strategies. In Figure 1, we depict the possible underlying etiology and management considerations based on timeline of the cytopenias being treated.
Early, prolonged and late grade 3-4 cytopenias following CAR T-cell therapy as reported in registry studies and real-world data
| . | Early (<30 d from infusion) . | Prolonged (30-90 d from infusion) . | Late (>90 d from infusion) . |
|---|---|---|---|
| CD19-directed CAR T-cells, pediatrics | |||
| ELIANA12 | Neutropenia: 53% | Neutropenia: 34% | |
| Thrombocytopenia: 41% | Thrombocytopenia: 27% | ||
| CD19-directed CAR T-cells, adults | |||
| ZUMA-113,14 | Neutropenia: 78% | Neutropenia: 11% | |
| Thrombocytopenia: 38% | Thrombocytopenia: 7% | ||
| Anemia: 43% | Anemia: 3% | ||
| JULIET15 | Neutropenia: 33% | Neutropenia: 24% | Grade 3-4 neutropenia: 0% |
| Thrombocytopenia: 28% | Thrombocytopenia: 41% | Thrombocytopenia: 38% | |
| Anemia: 39% | |||
| TRANSCEND16 | Neutropenia: 60% | Neutropenia: 7% | |
| Thrombocytopenia: 27% | Thrombocytopenia: 22% | ||
| Anemia: 37% | Anemia: 2% | ||
| ZUMA-217 | Neutropenia: 85% | Neutropenia: 16% | |
| Thrombocytopenia: 51% | Thrombocytopenia:16% | ||
| Anemia: 50% | Anemia: 12% | ||
| ZUMA-318 | Neutropenia: 27% | Neutropenia: 25% | |
| Thrombocytopenia: 30% | Thrombocytopenia: 18% | ||
| Anemia: 49% | Anemia: 7% | ||
| ZUMA-519 | Neutropenia: 33% | ||
| Thrombocytopenia: 15% | |||
| Anemia: 25% | |||
| BCMA-directed CAR T-cells, adults | |||
| KarMMa20 | Neutropenia: 89% | Neutropenia: 41% | |
| Thrombocytopenia: 52% | Thrombocytopenia: 48% | ||
| Anemia: 60% | |||
| CARTITUDE-121 | Neutropenia: 95% | Neutropenia: 29% | |
| Thrombocytopenia: 60% | Thrombocytopenia: 27% | ||
| Anemia: 68% | Anemia: 27% | ||
| Real-world studies | |||
| Jain et al4 | Neutropenia: 67% | Neutropenia: 20% | Neutropenia: 5% |
| Thrombocytopenia: 49% | Thrombocytopenia: 10% | Thrombocytopenia: 0% | |
| Anemia: 39% | Anemia: 7% | Anemia: 0% | |
| Juluri et al5 | Neutropenia: 46% | ||
| Thrombocytopenia: 34% | |||
| Anemia: 15% | |||
| Logue et al6 | Neutropenia: 39% | Neutropenia: 11% | |
| Thrombocytopenia: 51% | Thrombocytopenia: 28% | ||
| Anemia: 29% | Anemia: 15% |
| . | Early (<30 d from infusion) . | Prolonged (30-90 d from infusion) . | Late (>90 d from infusion) . |
|---|---|---|---|
| CD19-directed CAR T-cells, pediatrics | |||
| ELIANA12 | Neutropenia: 53% | Neutropenia: 34% | |
| Thrombocytopenia: 41% | Thrombocytopenia: 27% | ||
| CD19-directed CAR T-cells, adults | |||
| ZUMA-113,14 | Neutropenia: 78% | Neutropenia: 11% | |
| Thrombocytopenia: 38% | Thrombocytopenia: 7% | ||
| Anemia: 43% | Anemia: 3% | ||
| JULIET15 | Neutropenia: 33% | Neutropenia: 24% | Grade 3-4 neutropenia: 0% |
| Thrombocytopenia: 28% | Thrombocytopenia: 41% | Thrombocytopenia: 38% | |
| Anemia: 39% | |||
| TRANSCEND16 | Neutropenia: 60% | Neutropenia: 7% | |
| Thrombocytopenia: 27% | Thrombocytopenia: 22% | ||
| Anemia: 37% | Anemia: 2% | ||
| ZUMA-217 | Neutropenia: 85% | Neutropenia: 16% | |
| Thrombocytopenia: 51% | Thrombocytopenia:16% | ||
| Anemia: 50% | Anemia: 12% | ||
| ZUMA-318 | Neutropenia: 27% | Neutropenia: 25% | |
| Thrombocytopenia: 30% | Thrombocytopenia: 18% | ||
| Anemia: 49% | Anemia: 7% | ||
| ZUMA-519 | Neutropenia: 33% | ||
| Thrombocytopenia: 15% | |||
| Anemia: 25% | |||
| BCMA-directed CAR T-cells, adults | |||
| KarMMa20 | Neutropenia: 89% | Neutropenia: 41% | |
| Thrombocytopenia: 52% | Thrombocytopenia: 48% | ||
| Anemia: 60% | |||
| CARTITUDE-121 | Neutropenia: 95% | Neutropenia: 29% | |
| Thrombocytopenia: 60% | Thrombocytopenia: 27% | ||
| Anemia: 68% | Anemia: 27% | ||
| Real-world studies | |||
| Jain et al4 | Neutropenia: 67% | Neutropenia: 20% | Neutropenia: 5% |
| Thrombocytopenia: 49% | Thrombocytopenia: 10% | Thrombocytopenia: 0% | |
| Anemia: 39% | Anemia: 7% | Anemia: 0% | |
| Juluri et al5 | Neutropenia: 46% | ||
| Thrombocytopenia: 34% | |||
| Anemia: 15% | |||
| Logue et al6 | Neutropenia: 39% | Neutropenia: 11% | |
| Thrombocytopenia: 51% | Thrombocytopenia: 28% | ||
| Anemia: 29% | Anemia: 15% |
Considerations for etiologies and management of cytopenias after CAR T-cell infusion. ∗Remains speculative. #Antiviral prophylaxis is initiated early after CAR-T infusion and continued for 6 to 12 months. #Anti-PJP prophylaxis is started at day ∼28 until CD4+ cell count >200/mm3. #If the ANC has not recovered to >500/mm3 by day 7 to 10, consider institution of G-CSF. ANC, absolute neutrophil count; IEC-HS, hemophagocytic lymphohistiocytosis-like hyperinflammatory syndrome associated with immune effector cells; LD, lymphodepletion; LGL, large granular lymphocytosis; NGS, next generation sequencing; TA-TMA, transplant-associated thrombotic microangiopathy; TPO, thrombopoietin.
Considerations for etiologies and management of cytopenias after CAR T-cell infusion. ∗Remains speculative. #Antiviral prophylaxis is initiated early after CAR-T infusion and continued for 6 to 12 months. #Anti-PJP prophylaxis is started at day ∼28 until CD4+ cell count >200/mm3. #If the ANC has not recovered to >500/mm3 by day 7 to 10, consider institution of G-CSF. ANC, absolute neutrophil count; IEC-HS, hemophagocytic lymphohistiocytosis-like hyperinflammatory syndrome associated with immune effector cells; LD, lymphodepletion; LGL, large granular lymphocytosis; NGS, next generation sequencing; TA-TMA, transplant-associated thrombotic microangiopathy; TPO, thrombopoietin.
Infusion of CAR-T is almost universally preceded by LD chemotherapy, typically fludarabine and cyclophosphamide, which accounts for the majority of early cytopenias. LD promotes CAR T-cell expansion in vivo by inducing a lymphopenic environment, increasing homeostatic cytokines, and decreasing suppressive cells.23,24 LD is not myeloablative and recovery of blood counts is expected after chemotherapy.25,26 Most patients experience count nadir within the first week after CAR-T infusion, followed by rapid recovery. However, cytopenias commonly continue or recur beyond that expected from LD alone.
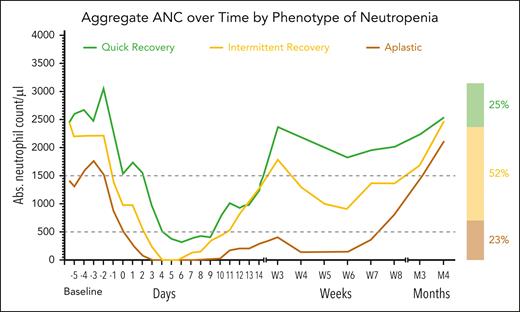
Cytopenia after CAR-T has been described as biphasic as many patients will experience a second decrease in ANC at a later timepoint,3 often occurring at week 3 or at the 1-month restaging visit. This “intermittent” neutrophil recovery is seen in many patients with prolonged cytopenia after CAR-T (Figure 2).27 A minority of patients experience an “aplastic” trajectory characterized by suboptimal count recovery post-nadir that is less responsive to growth factors. For these patients late cytopenia is common, and in the absence of other pathophysiologic processes, full recovery can occur months later.
Patterns of neutrophil recovery in lymphoma patients treated with CAR T-cell therapy. Reproduced from Rejeski et al.27
Patterns of neutrophil recovery in lymphoma patients treated with CAR T-cell therapy. Reproduced from Rejeski et al.27
Early cytopenia interventions
Before initiation of LD, we start with a comprehensive review of medications and replace myelosuppressive agents with alternatives when possible. For early cytopenias, we use supportive treatment with PRBC and platelet transfusions when indicated by institutional thresholds.
Infections can commonly occur in the first 30 days and could cause further myelosuppression and hematopoietic stem cell (HSC) exhaustion.28,29 Thorough evaluation and management of infections, including viral and fungal, are essential.7-9 Although there remains little prospective data on patients treated with CAR-T, our institutions have mirrored infectious prophylaxis to that used in patients undergoing blood or marrow transplantation (BMT). We begin bacterial and fungal prophylaxis on day 0, or at the time of first neutropenia, and continue until ANC count recovery. Antiviral prophylaxis is also initiated early after CAR-T infusion and continued for 6 to 12 months. Although Pneumocystis jirovecii pneumonia (PJP) can present early in severely immunocompromised patients, we typically initiate anti-PJP prophylaxis at day 30 and advocate for a CD4+ count driven approach to its discontinuation when CD4+ T-cell counts are >200, which can take from 1 to 2 years or longer.10 We commonly use trimethoprim-sulfamethoxazole for PJP prophylaxis, and consider pentamidine or atovaquone as acceptable alternatives if there are concerns for persistent cytopenias or drug allergy.
If the ANC has not recovered to >500/mm3 by day 7 to 10, we consider the institution of granulocyte colony stimulating factor (G-CSF), although the optimal timing of G-CSF intervention is ripe for additional investigation. There was theoretical concern that early use of G-CSF may worsen CRS and ICANS.30-32 Data remain inconclusive as some reports highlight the feasibility,33 whereas other small retrospective series raise concerns that G-CSF may increase duration of CRS34 or incidence of grade 3 to 4 CRS.35 Although previously reported in the context of BMT, it is unclear if G-CSF stimulation results in the skewing of CAR T-cell differentiation.36 Given the high risk for infection, these concerns must be balanced against the likely benefit of shortened duration of neutropenia.35 We commonly avoid the use of G-CSF in patients with ongoing CRS, unless there is concomitant severe infection in a setting of neutropenia. Of note, patients with CRS and ICANS have an elevation of serum granulocyte macrophage colony-stimulating factor (GM-CSF), and GM-CSF blockade abrogates similar immune-mediated complications in animal models, both of which inform our practice to avoid the use of GM-CSF in patients treated with CAR-T.30,32
Case 1 (continued)
The patient continued to have ongoing cytopenias with decreasing frequency with the need for PRBCs and platelets over time. Neutropenia was responsive to intermittent G-CSF. Bone marrow biopsy at 2 months was hypocellular without fibrosis or dysplasia and demonstrated normal cytogenetics without evidence of myelodysplastic syndrome (MDS) translocations using fluorescence in situ hybridization analysis, or clonal mutations by next generation sequencing. At 2 months, the patient required PRBCs every 2 weeks and weekly platelet transfusions, at which time he was started on eltrombopag 75 mg daily. Over the next 4 months, there was a gradual reduction in transfusion requirements. Eltrombopag was discontinued 6 months after therapy, and the patient maintained normal blood counts thereafter.
Prolonged (>30 and <90 days from infusion) cytopenias
The underlying pathophysiology of CAR-T mediated cytopenia beyond LD effects is not well understood and is likely complex. Immune driven suppression of the HSC and marrow microenvironment changes are believed to be major contributors to both prolonged and late cytopenia after CAR-T. The hyperimmune response after CAR-T infusion likely contributes to the marrow suppression, as incidence of severe cytopenia was associated with peak levels of inflammatory cytokines after CAR-T infusion in patients with ALL37; and prolonged cytopenia was associated with grade 3 to 4 CRS or ICANS in both standard of care and trial settings with CD19- and BCMA-directed CAR-T.4,5
Preceding cytopenias related to disease or prior chemotherapy, present at the time of CAR-T infusion, are commonly associated with cytopenias beyond day 30,4,5 highlighting the impact of marrow reserve going into the therapy. Patients with a prior allogeneic BMT may have both a preexisting proinflammatory state and decreased marrow reserve, predisposing to cytopenia.4,38 Patients may also have residual or recurrent disease in the marrow with a varying likelihood depending on the primary disease (ie, BCL vs ALL vs MM) and disease status at the time of CAR-T infusion (relapsed vs refractory). Patients with high marrow disease have low levels of hematopoietic progenitor cells that require time to mature and expand.6 Finally, the costimulatory domain of the CAR may impact count recovery.4
Additional etiologies to consider in patients with early and prolonged cytopenias include infections and immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS). Infections may cause myelosuppression via direct and immune-mediated suppression of HSC.28,29 Although generally rare, higher rates of IEC-HS have been reported with specific CAR constructs, particularly those targeting CD22.39-42 Suspicion for IEC-HS should be raised in cases in which previously resolved CRS is followed by a rapid increase in ferritin (often >10 000 ng/mL, sometimes >100 000 ng/mL), recurrence of fever, and new onset of severe cytopenia, particularly when temporally related to increasing transaminases, hypertriglyceridemia, coagulation abnormalities, new splenomegaly, or other organ dysfunction.39-42 Demonstration of phagocytosis in the bone marrow biopsy will substantiate this rare etiology of cytopenia.
Biomarkers consistent with early and prolonged cytopenias
The wide recognition of cytopenias beyond the expected effect of LD has prompted interest in elucidation of predictive biomarkers. Serum ferritin and C-reactive protein tests are widely available in clinical labs and values are associated with proinflammatory cytokines from disease and CAR-T activity. Higher peak C-reactive protein and ferritin after CAR-T infusion correlated with lower probability of complete white blood cell count, hemoglobin, platelet, and neutrophil count recovery.4 Analysis of specific inflammatory cytokines have led to conflicting results, implicating general inflammation rather than specific and targetable markers. As mentioned above, IL-6 and multiple other cytokines are associated with cytopenias,37 whereas another study found none of the tested cytokines including IL-6 were statistically correlated with count recovery despite some suggestive trends.4 In yet another study, higher peak IL-6 was associated with lower counts at day 28 and higher trough levels of transforming growth factor beta with higher day 28 platelets and neutrophils.5 These differences are attributable to the variations in the products evaluated and sample size limitations in each study. Nevertheless, they substantiate the possibility of cytokine-mediated dysfunction of the stem cell compartment.
Interventions for prolonged cytopenia
When managing prolonged or late cytopenia, it is important to recognize that in the absence additional pathological process, gradual recovery is the likely trajectory even without intervention.4,10 Vigilance should be maintained for alternative processes such as active primary disease in the marrow and secondary myeloid malignancies.
Patients with prolonged severe neutropenia or those with active infection may be treated with G-CSF. For example, in patients with initial count recovery, and then later biphasic drop in neutrophils to <750/mm3, we often use daily G-CSF until ANC is >1500/mm3. Transient responses in these cases are common and suggest that gradual improvement and decreased dependence on G-CSF is likely to occur over weeks. A complete lack of responsiveness to G-CSF may prompt bone marrow biopsy. In most cases the marrow is hypocellular and without dysplasia, consistent with cytopenia related to CAR-T mediated inflammation and prior therapy. However, for prolonged cytopenia we also consider a bone marrow aspirate when there is a high likelihood of primary disease progression/relapse, evidence of marrow fibrosis or lineage dysplasia before CAR-T, or evidence of IEC-HS.
IEC-HS is rare but if suspicion is high, treatment with anakinra and high-dose steroids may be considered.40-43 Anecdotal reports suggest that refractory IEC-HS may respond to ruxolitinib or immunosuppressive chemotherapeutics such as cyclophosphamide or etoposide.42 However, the potential for cytotoxic impact on both HSC and CAR-T should be considered when selecting interventions. Future studies should explore the use of emapalumab.44
For prolonged severe thrombocytopenia requiring transfusion support well beyond day 30 without clear etiology other than CAR-T, we consider the use of thrombopoietin-receptor agonists (eltrombopag 75 mg daily with consideration for titration to 150 mg or romiplostim 10 μg/kg per week) for patients at high risk of bleeding and hemorrhage.45,46 We favor stopping thrombopoietin-receptor agonists once platelets recover or if no response is observed in 4 to 6 weeks after initiation. Counts often recover without intervention. Therefore, if transfusion requirements are not severe, we often elect to reserve the use of thrombopoietin-receptor agonists for late cytopenia or even forgo their use completely. Evidence for thrombopoietin-receptor agonists after CAR-T is derived from retrospective studies in which some patients achieved transfusion independence within 60 days after thrombopoietin-receptor agonist use.45-47 Similar to trilineage improvement in hematopoiesis reported with thrombopoietin-receptor agonists in bone marrow failure syndromes, we have seen improvement in counts in all 3 hematopoietic lineages with this intervention in post-CAR-T setting.46,48 Thrombopoietin-receptor agonists induce downstream activation of intracellular signaling cascades (JAK-STAT and MAPK pathways) and support trilineage hematopoiesis in chronic inflammatory conditions.48,49 As proinflammatory suppression of HSC is likely at play contributing to the CAR-T-related cytopenias, the role of thrombopoietin-receptor agonists needs to be explored in a systematic manner.
Case 2
A 63-year-old woman received idecabtagene vicleucel (ide-cel) CAR-T as sixth-line treatment (including autologous transplant) for refractory IgA-kappa MM having 56% plasma cell involvement of the marrow at the time of leukapheresis for CAR-T manufacture. She received DCEP (dexamethasone, cyclophosphamide, etoposide, and cisplatin)-bridging chemotherapy with subsequent hematologic toxicity requiring transfusions of both red blood cells and platelets immediately before the initiation of LD. After ide-cel infusion, she experienced maximum Grade 1 CRS and Grade 2 ICANS treated with tocilizumab and dexamethasone. ANC did not recover to >500/mm3 despite G-CSF, and the patient was entirely dependent on PRBC (2-3 times weekly) and platelet (2 times weekly) transfusions. Eltrombopag was initiated at 6 weeks after therapy, however transfusion dependence continued. Bone marrow biopsy at month 3 was profoundly hypocellular with a marked decrease in myeloid and erythroid precursors without increase in plasma cells or blasts.
Late (>90 days from infusion) cytopenias
Cytopenias improve over time for many patients but can persist late beyond 90 days.4,5 At this time, many patients have reestablished care with their referring doctors, whereas we advocate patients continue restaging visits for at least 2 years from CAR-T at the CAR-T treatment center whenever possible. In a setting of prolonged hypogammaglobulinemia, this poses a high risk of acquiring infections. The evaluation and interventions as described for prolonged cytopenia remain appropriate including the avoidance of medication that could suppress the marrow, workup for occult infection, and bone marrow aspirate and biopsy to evaluate for alternate causes. We also extend bacterial and fungal infection prophylaxis for patients with severe neutropenia. In our practice in adult patients, fluconazole or micafungin is the common choice for antifungal prophylaxis in most patients. We also consider an azole with mold-coverage for select patients who have prolonged or late severe neutropenia.
The severity and persistence described in case 2 above is rare. In most cases, late cytopenia may continue to be accompanied by a hypoplastic marrow without evidence of fibrosis, dysplasia, or clonal hematopoiesis of indeterminate potential (CHIP) mutations, and in our experience, these patients are likely to recover counts, albeit slowly, with decreasing need for transfusion and growth factors leading to complete independence occurring as late as 6 to 12 months. The incidence of cases with severe persistent aplasia is rare in adults and attribution purely to CAR-T is often clouded by marrow dysfunction from previous transplant or therapy, such as that described in case 2. If immune-mediated dysfunction is suspected, immunosuppressive agents could be considered. Autologous stem cell boost is described in detail below, and is preferred over allogeneic BMT as treatment for severe transfusion–ependent late cytopenia in the absence of secondary malignancy or recurrent primary disease. Consideration of allogeneic BMT should be made in rare cases in which complete aplastic anemia occurs with little evidence of hematopoiesis, as seen in case 2, and is discussed later in this manuscript. Lastly, we have anecdotally encountered cases complicated by large granular lymphocytosis without evidence of CAR-T, and we have used immunosuppressive treatment in these cases with some alleviation of ongoing cytopenia.
The persistence of cytopenia late after CAR-T also raises concerns about a secondary leukemia or clonal marrow pathology. Secondary malignancies, including both solid tumors and hematological cancers, have been reported at rates of 2% to 16%.4,50-53 Older patients may have CHIP predisposing them to emergence of MDS after CAR-T.54 We speculate that immune processes may differ in prolonged cytopenia that is mediated by cytokine stunning and gradually recovers, whereas late cases of severe trilineage aplasia may be caused by persistent CAR activation, which, in turn, causes ongoing immune-mediated mature blood cell destruction. It is unclear if the CAR-T-mediated inflammation or immunosuppression accelerates the emergence of therapy-related myeloid neoplasms, however within the first 90 days after CAR-T we have seen rapid onset of MDS in patients with preceding CHIP mutations.55 Finally, there is a remote risk of T-cell leukemia arising from viral vector-based gene integration disrupting tumor suppressor genes or activation of proto-oncogenes, which could theoretically impact marrow function.56,57 There are 2 cases reported thus far with CAR19 T-cell lymphoma (despite remission of B-cell malignancy) in which CAR19 T-cells were manufactured using the piggyBac transposon system, a nonviral transduction technique for CAR-T manufacturing.57 Thus far, this has not been reported with the commercially available CAR products.
Case 2 (continued)
Transfusion dependency continued with severe pancytopenia. At 4 months after CAR-T, the patient was treated with a stem cell boost, consisting of ∼10 × 106 cryopreserved mobilized HSC, previously collected for autologous transplant. Within 2 weeks she was no longer transfusion or growth factor dependent and remains in stringent complete response 1 year later.
Stem cell boost for transfusion-dependent multilineage cytopenias
Prolonged and late transfusion-dependent cytopenias in >1 lineage, present a clinical challenge with a paucity of data to guide management. Beyond the thrombopoietin-directed strategies described above, HSC boost has been used to address persistent or life-threatening cytopenias.58,59 This strategy is only feasible if stem cells from a prior autologous or an allogeneic BMT were persevered and are available for use. The concept of stem cell boost has been long used in BMT-related cytopenias or poor graft function to restore hematopoiesis.60,61 To address severe cytopenias related to CAR-T, both CD34+ selected and unmanipulated stem cells have been used without antecedent conditioning chemotherapy with hematopoietic improvement in many patients. Stem cell products may be contaminated with the primary malignant cells, therefore, caution is warranted as relapse was noted in a patient with mantle cell lymphoma.59 Although all reports to date are single-arm and noncomparative, using stem cell boost decreases sequalae of cytopenia after CAR-T and potentially improves quality of life. Future studies are likely to highlight the optimal indication, timing, and stem cell product for this intervention.
Case 3
A 31-month-old female child presented for CAR-T evaluation for relapsed KMT2A-rearranged B-ALL in the bone marrow and CNS, 8 months after haploidentical allogeneic BMT in CR2 induced with blinatumomab. Before relapse, she had tolerated her transplant course well with no graft versus host disease (GVHD) and the only significant organ toxicity being acute kidney injury manifested as elevated creatinine, proteinuria, and hypertension that resolved by 2 months after BMT. Adequate donor T-cell reconstitution enabled collection and manufacture of tisagenlecleucel (100% donor T-cells at the time of collection). She achieved CR3 after 2 cycles of bridging therapy and underwent LD followed by CAR-T infusion. She developed grade 3 CRS and ICANS, which were successfully treated with tocilizumab and dexamethasone, and both resolved within 4 weeks. However, her ANC remained persistently below 100/mm3 for 6 weeks after CAR-T, prompting the initiation of G-CSF support, which continued beyond 90 days after CAR-T. Although transfusion requirements improved after resolution of CRS symptoms, she continued to require PRBC and platelets every 2 weeks beyond 90 days. Molecular disease evaluations for recurrent B-ALL remained negative.
Pediatric-specific considerations for persistent cytopenias after CAR-T
In the setting of prolonged cytopenias after CAR-T, there are several etiologies and treatment considerations that are unique to, or more commonly arise in, pediatric patients. First, in young infants and other pediatric patients with certain cytogenetic drivers of B-ALL clones (eg, KMT2A rearrangements), lineage switch can occur after CAR-T.62-64 Thus, occult myeloid malignancy after lineage switch could be an underlying cause of prolonged cytopenias.65 Close attention to marrow morphology, including dysplastic features and fibrosis, along with broad cytogenetic, immunophenotype, and molecular screening including next-generation sequencing of B-cell receptor rearrangements for minimal residual disease tracking in ALL is warranted.52 Second, owing to increased thymic activity and a uniquely persistent immune environment in children,66,67 sustained immune dysregulation leading directly to hematopoietic suppression or immune-mediated mature blood cell destruction may be more likely in pediatric patients. Third, pediatric patients are more likely to have undergone previous allogeneic BMT, with special considerations described below.3 Whether or not a patient had an antecedent BMT, most pediatric patients would be considered candidates for BMT after CAR-T, either to consolidate B-ALL remission or to resolve cytopenias in extreme cases.68 Therefore, prolonged use of growth factors are often deferred in pediatric patients. 45,69,70 Furthermore, there are concerns that myeloid growth factors might exacerbate CRS,17,71 and thrombopoietin mimetics might trigger myeloid clonal evolution in other diseases.72 In contrast to adults, for BMT-naïve pediatric patients with transfusion dependence or severe neutropenia requiring G-CSF therapy late after CAR-T infusion (beyond 90 days), allogeneic BMT with best available donor should be considered.
Cytopenias after CAR-T in patients who have undergone previous BMT
After the severity of CRS, prior BMT is the next most consistent risk factor correlating with prolonged cytopenias after CAR-T.3 In pediatric patients who relapse with B-ALL after allogeneic BMT, the donor graft can be significantly damaged by the infiltrative effects of the relapse itself and by salvage chemotherapy.73 In patients who receive CAR-T after a post-BMT relapse, prolonged myelosuppression from lymphodepleting chemotherapy or from interferon-gamma driven hematopoietic suppression during CRS may lead to prolonged graft dysfunction or even graft failure.74,75 In addition, BMT-associated complications may recrudesce after the intensive immune suppression of LD chemotherapy and the immune activation that occurs during CRS. Reactivation of viruses, including human herpesvirus 6, cytomegalovirus, and Epstein-Barr virus, driven by immune suppression from LD and CRS76 can directly suppress hematopoietic output. The use of select antiviral agents to control these infections pre- or post-CAR-T infusion, including ganciclovir and rituximab may also exacerbate cytopenias.77,78 GVHD may arise in patients who receive true allogeneic donor CAR-T,79,80 and in such patients GVHD sequelae could also drive prolonged cytopenias.81 Finally, transplant-associated thrombotic microangiopathy (TA-TMA) may be a specific consideration in patients with prolonged transfusion-dependent anemia and thrombocytopenia but neutrophil recovery, with additional evidence including elevated immature platelet fraction, elevated reticulocyte counts, and hemolytic markers.82 Patients who meet this description should have a formal assessment for TA-TMA, including assessment of hypertension and current need for antihypertensives, proteinuria, and increased terminal complement activation.83
For patients who are post-BMT with late cytopenias persisting beyond 90 days after CAR-T, and that appear to be because of bone marrow hypocellularity and donor graft dysfunction, strong consideration should be given to providing a stem cell boost if the original donor is available or if leftover T-cell depleted CD34+ cells are available. Small case series show that allogeneic stem cell boosts can be effective in treating post-CAR-T cytopenias.58,59 If a new collection from the donor is required, we and others would use an ex vivo T-cell depleted (CD34 selection or TCRαβ T-cell depletion) mobilized peripheral stem cell source for this boost, thus avoiding repeat bone marrow harvest from the original donor and alloimmune complications from infusion of donor T-cells. In patients with adequate organ function after initial BMT and CAR-T, consideration should be given to second allogeneic BMT in lieu of a stem cell boost, particularly if availability of the original donor is limited.
Case 3 (continued)
Stem cell boost was considered, however the patients transfusion requirements decreased over time to when neither platelet nor red blood cell transfusions were required after 6 months. The patient remains in remission 2 years after therapy.
Conclusion
Cytopenias after CAR-T are common (15%-95%) and often persistent (2%-45%). Alternative, non-CAR-T specific etiologies must be considered. In the absence of these, the underlying biology remains elusive, although cytopenias are associated with the severity of inflammatory toxicities, baseline cytopenias, previous BMT, and marrow disease burden, suggesting a cytokine–mediated stem cell quiescence. As the biology of these cytopenias is better understood, specific treatment strategies aimed at abrogating immune impacts on marrow or improving stem cell fitness may emerge. How we treat these cytopenias is first to recognize that they often resolve over time absent alternative pathology and next, to use management strategies based upon their severity and timing after CAR-T.
Authorship
Contribution: T.J., T.S.O., and F.L.L. designed the study, analyzed the data, wrote the manuscript, and approved the final draft.
Conflict-of-interest disclosure: T.J. has institutional research support from CTI Biopharma, SyneosHealth, Incyte; advisory board participation with Care Dx, Bristol Myers Squibb, Incyte, AbbVie, CTI, and Kite; education activity with Oncology APP, Binaytara Foundation, and ASH Highlights. T.S.O. has a consulting agreement with bluebird bio, Vertex, Medexus. F.L.L. has scientific advisory role/consulting fees from A2, Allogene, Amgen, bluebird bio, BMS/Celgene, Calibr, Caribou, Cellular Biomedicine Group, Cowen, Daiichi Sankyo, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Group, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, Umoja; contracts for service from Kite Pharma (institutional), Allogene (institutional), CERo Therapeutics (institutional), Novartis (institutional), bluebird bio (institutional), BMS (institutional), National Cancer Institute, Leukemia and Lymphoma Society; several patents held by the institution in their name (unlicensed) in the field of cellular immunotherapy; education or editorial activity in Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, Society for Immunotherapy of Cancer.
Correspondence: Tania Jain, Division of Hematological Malignancies and Bone Marrow Transplantation, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, 1650 Orleans St Room 3M-88, Baltimore, MD 21202; e-mail: tjain2@jhmi.edu; Timothy S. Olson, Department of Pediatrics, Children’s Hospital of Philadelphia, Colket Translational Research Building, Room #3010, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: olsont@chop.edu; and Frederick L. Locke, Department of Blood and Marrow Transplant and Cellular Immunotherapy, Moffitt Cancer Center, 12902 USF Magnolia Dr, BMTCI CSB-7, Tampa, FL 33612; e-mail: frederick.locke@moffitt.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal