Key Points
HLA-haploidentical BMT with PTCy as upfront therapy in patients with SAA results in survival >90% and low morbidity/mortality.
More than 35% of patients were from underrepresented race/ethnic backgrounds, demonstrating accessibility to those lacking matched donors.
Abstract
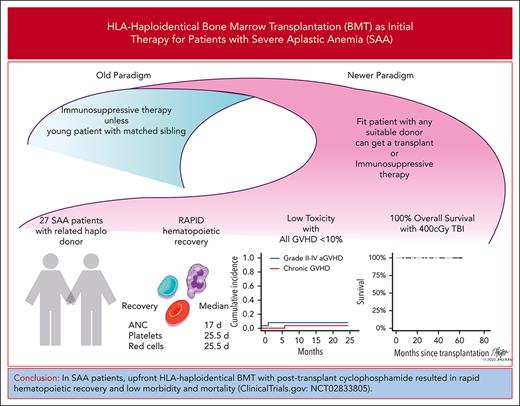
Severe aplastic anemia (SAA) is a marrow failure disorder with high morbidity and mortality. It is treated with bone marrow transplantation (BMT) for those with fully matched donors, or immunosuppressive therapy (IST) for those who lack such a donor, which is often the case for underrepresented minorities. We conducted a prospective phase 2 trial of reduced-intensity conditioning HLA-haploidentical BMT and posttransplantation cyclophosphamide (PTCy)-based graft-versus-host (GVHD) prophylaxis as initial therapy for patients with SAA. The median patient age was 25 years (range, 3-63 years), and the median follow-up time was 40.9 months (95% confidence interval [CI], 29.4-55.7). More than 35% of enrollment was from underrepresented racial/ethnic groups. The cumulative incidence of grade 2 or 4 acute GVHD on day 100 was 7% (95% CI, not applicable [NA]-17), and chronic GVHD at 2 years was 4% (95% CI, NA-11). The overall survival of 27 patients was 92% (95% CI, 83-100) at 1, 2, and 3 years. The first 7 patients received lower dose total body irradiation (200 vs 400 cGy), but these patients were more likely to have graft failure (3 of 7) compared with 0 of 20 patients in the higher dose group (P = .01; Fisher exact test). HLA-haploidentical BMT with PTCy using 400 cGy total body irradiation resulted in 100% overall survival with minimal GVHD in 20 consecutive patients. Not only does this approach avoid any adverse ramifications of IST and its low failure-free survival, but the use of haploidentical donors also expands access to BMT across all populations. This trial was registered at www.clinicaltrials.gov as NCT02833805.
Introduction
Acquired severe aplastic anemia (SAA) is a rare, life-threatening hematopoietic stem cell disorder that manifests with pancytopenia.1,2 Bone marrow failure results from autoimmune destruction of hematopoietic stem cells.1 Without definitive treatment, mortality from SAA at 2 years approaches 70%. Fungal infections are the leading cause of death; however, hemorrhage, evolution to clonal disease (myelodysplastic syndromes [MDS], leukemia, and paroxysmal nocturnal hemoglobinuria [PNH]), along with transfusional iron overload are additional causes of severe morbidity and mortality.
Immunosuppressive therapy (IST) has been the standard frontline treatment for SAA for decades, except for patients aged <40 years with a suitable HLA-matched sibling donor for bone marrow transplantation (BMT).3,4 The standard IST regimen (antithymocyte globulin [ATG] and cyclosporine) has undergone modest improvements to decrease the time to hematologic response,4,5 but longer-term durability remains relatively unchanged even with the addition of eltrombopag. The hematopoietic response after IST is ∼70% to 80% including recent augmentation of IST with the use of eltrombopag.3,4,6 Failure-free survival, or patients alive and in remission without clonal disease >10 years after IST, remains <40% in most series.1,3,7-9 Patients for whom IST fails are often required to receive a BMT.
In contrast, innovations in BMT in recent decades have steadily improved outcomes. Standard platforms exist with the goal of rapid hematopoietic reconstitution, representing a cure for SAA.10 Long-term historical survival after BMT is ∼90% in patients aged <20 years,11,12 and 75% in older patients.10,12 At most centers, because of concerns for morbidity and mortality, BMT with an unrelated or HLA-haploidentical related donor is currently reserved for use after the failure of IST.13-19
Unfortunately, complications after IST may negatively affect BMT outcomes and donor options. The most common MDS-associated clonal cytogenetic abnormality after IST for SAA is monosomy 7, which has notoriously poor outcomes with BMT.20 IST failure can also enhance rates of donor-specific antibodies and limit mismatched related donor options. Long-term immunosuppressive use with cyclosporine can also limit the quality of life after IST, with orthopedic complications such as avascular necrosis, organ complications including renal failure, or pulmonary fungal infections.21 All of these issues could preclude BMT after IST as an option for an individual with SAA.
In this era, goals of upfront therapy should include availability to all patients and avoidance of long-term complications. When the use of upfront IST has the aforementioned complications, BMT should not be reserved as salvage therapy alone in 2023 and beyond. Here, we report the outcomes of patients with SAA who were uniformly treated and had an alternative donor BMT as their initial therapy for SAA.
Methods
Trial design and conduct
This trial was conducted at a single center in the United States for patients with SAA. It was a single-arm, phase 2 clinical trial to evaluate both the feasibility and safety of initial BMT therapy for patients with SAA. The trial was approved by the institutional review board and its conduct adhered to the Declaration of Helskinki.
Patients
Patients were enrolled with a confirmed diagnosis of SAA, either from initial diagnosis or follow-up assessments; SAA was defined as bone marrow hypocellularity relative to the patient’s age (normocellularity is 100 minus the patient’s age in years) with 2 of the 3 following criteria (in peripheral blood): neutrophils <0.5 × 109/L, platelets <20 × 109/L (without transfusions), or reticulocyte count <60 × 109/L. Disease could be acquired or inherited but not Fanconi anemia or a short telomere disorder. Moreover, patients could not have a suitable fully matched related (6/6 match for HLA-A and HLA-B at an intermediate or high resolution and HLA-DRB1 at a high resolution using DNA-based typing) donor if aged <25 years. Unrelated searches were not routinely performed because related donors are prioritized institutionally. When multiple haploidentical donors were available, the chosen donor was prioritized, as previously published.22 Patients were excluded if previous administration of IST for SAA had occurred. Patients needed adequate organ function, as previously reported.23 Additional eligibility criteria included an adequate performance status (Eastern Cooperative Oncology Group score of 0 or 1; Karnofsky or Lansky ≥60%) and organ function, as previously reported23; the ability to provide informed consent (and assent as appropriate for minors); and the presence of an eligible, related, HLA-haploidentical donor. A karyotype with monosomy 7 was specifically excluded because it is considered to be consistent with MDS or leukemia. Clonality was assessed via metaphase karyotyping and PNH flow cytometry for all patients. Next-generation sequencing was not required by the protocol nor was formal genetic testing for inherited predisposition; both were inconsistently performed between adult and pediatric clinicians. All patients gave informed consent as approved by institutional review boards at Johns Hopkins, initially on clinical trial and subsequently at retrospective review. This study in which 20 of the current patients were treated was registered as NCT02833805. The study was opened in August 2016 and completed accrual in July 2020. After protocol completion, 7 additional patients were treated from August 2020 to August 2022, following the protocol procedures in an identical fashion.
Treatment
A suitable donor was defined as an available HLA-haploidentical relative of the patient, including a biological parent, sibling or half sibling, child, uncle, aunt, first cousin, or extended relative. The presence of donor-specific antibodies (at a level of mean fluorescence intensity >1000 via solid phase immunoassay) was an exclusion criterion.
All patients received fresh harvested bone marrow with a target yield of 4 × 108 nucleated marrow cells per kg of the recipient ideal body weight (IBW)24 and were infused with the bone marrow on day 0. Marrow nucleated cells per kg dose was calculated as previously described.24
The conditioning regimen was as previously published.23 Rabbit ATG (rATG; Thymoglobulin) was dosed at 0.5 mg/kg on day −9 and dosed at 2 mg/kg on days −8 and −7 IV. Fludarabine was administered at 30 mg/m2 IV daily for 5 days, from day −6 to day −2 (total dose received, 150 mg/m2). Cyclophosphamide was given at 14.5 mg/kg IV daily for 2 days from day −6 to day −5 and administered as a 1-to-2–hour infusion (total dose received, 29 mg/kg), and total body irradiation (TBI) was delivered in a single fraction of 200 cGy on day −1. After the initial 7 patients were treated, an institutional review board–approved protocol amendment augmented this to a single fraction of 400 cGy on day −1 in an attempt to enhance engraftment (as applied in other diseases25). The marrow graft was infused on day 0. Granulocyte colony-stimulating factor was given on day +5 at 5 μg/kg per day and was continued until absolute neutrophil count (ANC) was maintained at >1.5 ×109/L for 3 consecutive days. In addition to a contribution from rATG, posttransplant graft-versus-host disease (GVHD) prophylaxis included posttransplant cyclophosphamide (PTCy) administered at 50 mg/kg per day IV on days +3 and +4, mycophenolate mofetil orally given at a dose of 15 mg/kg three times per day up to 1 g three times per day (max dose 3000 mg per day) from day +5 to day +35, and tacrolimus orally or IV was given starting day +5 to maintain a level of 10 to 15 ng/mL. Tacrolimus was discontinued without taper for patients without GVHD, initially, on day +365 in the first 10 patients and then on day +180 in all remaining patients.
Blood product replacement and other supportive care measures were per institutional practices, as were prophylactic and empiric antibiotics, antifungal prophylaxis, Pneumocystis jirovecii pneumonia prophylaxis, and IV immunoglobulin. Patients were monitored for cytomegalovirus (CMV) reactivation via weekly measurement of CMV. If a patient had evidence of viral reactivation, it was managed per the discretion of the oncologist and infectious disease physicians. Viral prophylaxis during the period of this study was valacyclovir or acyclovir (not letermovir). Human herpes virus 6 levels were monitored weekly until day +60. In the event of development of either acute or chronic GVHD, therapy was per the standard of care. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Outcomes
This single-arm phase 2 clinical trial evaluated both the feasibility and safety of initial BMT therapy for patients with SAA. Patient level feasibility required transplantation with engraftment, and survival at 1 year. Secondary end points included overall survival (OS) at 1 year after BMT, neutrophil and platelet recovery, graft failure (primary and secondary), grades 2 or 4 acute GVHD and chronic GVHD, immune reconstitution, and the rate of specific infectious complications (CMV viremia and disease and Epstein-Barr virus [EBV] viremia with or without posttransplant lymphoproliferative disease) within the first year after transplant.
Neutrophil recovery was defined as an ANC > 0.5 × 109/L measured for 3 consecutive measurements on different days. Platelet recovery was defined as a platelet count > 20 × 109/L for 7 days without transfusion. Red blood cell recovery was defined as a hemoglobin (Hb) level > 7 g/dL for 7 days without transfusion. Response was classified per the National Institutes of Health criteria.26 Complete response (CR) at 6 and 12 months was defined as Hb levels > 10 g/L, ANC > 1.0 × 109/L, platelets > 100 × 109/L, and no evidence of clonal evolution. Transfusion burden for red blood cells and platelets was calculated from the 6 weeks before the start of preparation until transfusion independence. Traditional metrics were followed for transfusional support, with transfusions provided when Hb was <7 g/dL and platelet counts ranged from <10 000 to 20 000, unless a procedure or active bleeding necessitated a threshold of 50 000.
Patients had donor chimerism measured in the peripheral blood or bone marrow on days 28, 56, 180, and 360. Whole blood and T-cell (CD3) chimerism were required. Primary graft failure was defined by the lack of neutrophil recovery by day +56 after BMT or failure to achieve at least 5% donor chimerism (whole blood or marrow) on any measurement up to and including day +56; patients could have met this definition before day +56. Secondary graft failure was defined as any 1 of the following: initial neutrophil recovery followed by sustained subsequent decline in ANC to < 0.5 × 109/L for 3 consecutive measurements on different days; or initial whole blood or marrow donor chimerism ≥5% but then declining to <5% upon subsequent measurements; or second infusion/transplant given for graft failure. The management beyond any documented graft failure was not specified by this protocol.
Acute GVHD was graded using consensus grading. The time of onset of grades 2 to 4 and grades 3 or 4 acute GVHD as well as the maximum grade achieved were recorded. Chronic GVHD was assessed based on the 2014 National Institutes of Health consensus criteria.
All patients were followed up at least yearly after day 365 per standard BMT procedures, with clinical visits to assess for recurrent infections, return to previous functional status, ongoing donor chimerism, and blood count normalization. Immune reconstitution laboratory visits were not mandated by the protocol.
Statistical methods
The primary analysis evaluated both the feasibility and safety of BMT as initial therapy for SAA. Feasibility and safety were monitored continually using Bayesian monitoring rules. The monitoring rule considered each patient as “feasible” based on 3 benchmarks: transplantation, engraftment, and survival at 1 year. The reference value for monitoring feasibility was based on 1-year survival of upfront nonmyeloablative SAA transplants between July 1999 and June 2012 from the Center for International Blood and Bone Marrow Transplant Research. Feasibility is reported with an exact 95% confidence interval (CI). Safety stopping rules were based on mortality, graft failure, and acute and chronic GVHD. Additional discussions of the decision rules and safety monitoring can be found in the supplemental Methods, available on the Blood website. OS and median follow-up were reported using the Kaplan-Meier and reverse Kaplan-Meier methods, respectively. The cumulative incidence for hematologic recovery outcomes and GVHD were estimated using competing risk methods. Analyses were performed using SAS version 9.4 and R version 3.6.
Results
The clinical trial was opened for enrollment in August 2016 and continued through July 2020. In total, 20 patients were treated in the trial; thereafter, 7 patients were treated per our internal standard of care in accordance with the trial procedures. There were no screen failures due to the presence of donor-specific antibodies; however, 3 patients were excluded because of the lack of any suitable related haploidentical donor. Median follow-up for all patients was 40.9 months (95% CI, 29.4-55.7); the minimum and maximum follow-up was 6.0 months and 73.8 months, respectively. Table 1 shows the demographics of the patients and their donors. Of the 27 patients who received transplants, 14 (52%) were male, with 37% of the cohort self-reported as being non-White. There were 3 (11%) patients who were Asian/Pacific Islanders, 6 (21%) who were Black, and 1 (3%) who was mixed or other race, reporting as Hispanic. The median age at enrollment was 25 years (range, 3-63 years; interquartile range, 17-52 years). The median age of the haploidentical donors was 30 years (range, 13-56 years; interquartile range, 26-44 years).
Baseline characteristics of patients who received transplant
| . | Total (N = 27), n (%) . |
|---|---|
| Sex | |
| Female | 13 (48) |
| Male | 14 (52) |
| Race and ethnicity | |
| Non-Hispanic White | 17 (63) |
| Hispanic White | 0 |
| Non-Hispanic Black | 5 (19) |
| Hispanic Black | 1 (3) |
| Asian/Pacific Islander | 3 (11) |
| Native American | 0 |
| >1 race | 1 (3) |
| Unknown | 0 |
| Age, y | |
| Mean (SD) | 32 (19) |
| Median (range) | 25 (3-63) |
| Age, y | |
| <10 | 2 |
| 10-19 | 6 |
| 20-29 | 7 |
| 30-39 | 1 |
| 40-49 | 2 |
| 50-59 | 6 |
| 60-69 | 3 |
| Very severe AA diagnosis (ANC <0.2 × 109/L) | 14 (52) |
| SAA diagnosis (ANC <0.5 × 109/L) | 13 (48) |
| Clonality at baseline (PNH clone or molecular data including karyotype) | 22 (81) |
| Documented inherited predisposition to AA | 1 (3) |
| Interval from diagnosis to transplant, d | |
| Mean (SD) | 91 (55) |
| Median (range) | 78 (12-249) |
| Donor age, y | |
| Mean (SD) | 33 (12) |
| Median (range) | 30 (13-56) |
| Donor relationship | |
| Haplo sibling | 7 (26) |
| Haplo parent | 6 (22) |
| Haplo child | 7 (26) |
| Haplo second-degree relative (cousin, niece, or nephew) | 7 (26) |
| Donor sex | |
| Female | 16 (59) |
| Male | 11 (41) |
| . | Total (N = 27), n (%) . |
|---|---|
| Sex | |
| Female | 13 (48) |
| Male | 14 (52) |
| Race and ethnicity | |
| Non-Hispanic White | 17 (63) |
| Hispanic White | 0 |
| Non-Hispanic Black | 5 (19) |
| Hispanic Black | 1 (3) |
| Asian/Pacific Islander | 3 (11) |
| Native American | 0 |
| >1 race | 1 (3) |
| Unknown | 0 |
| Age, y | |
| Mean (SD) | 32 (19) |
| Median (range) | 25 (3-63) |
| Age, y | |
| <10 | 2 |
| 10-19 | 6 |
| 20-29 | 7 |
| 30-39 | 1 |
| 40-49 | 2 |
| 50-59 | 6 |
| 60-69 | 3 |
| Very severe AA diagnosis (ANC <0.2 × 109/L) | 14 (52) |
| SAA diagnosis (ANC <0.5 × 109/L) | 13 (48) |
| Clonality at baseline (PNH clone or molecular data including karyotype) | 22 (81) |
| Documented inherited predisposition to AA | 1 (3) |
| Interval from diagnosis to transplant, d | |
| Mean (SD) | 91 (55) |
| Median (range) | 78 (12-249) |
| Donor age, y | |
| Mean (SD) | 33 (12) |
| Median (range) | 30 (13-56) |
| Donor relationship | |
| Haplo sibling | 7 (26) |
| Haplo parent | 6 (22) |
| Haplo child | 7 (26) |
| Haplo second-degree relative (cousin, niece, or nephew) | 7 (26) |
| Donor sex | |
| Female | 16 (59) |
| Male | 11 (41) |
AA, aplastic anemia; haplo; haploidentical; SD, standard deviation.
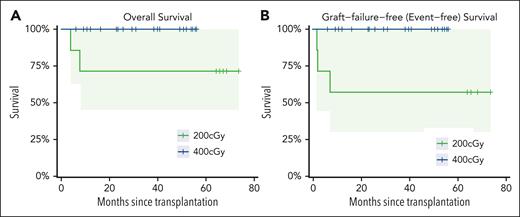
Twenty-five patients were alive, and 24 patients had sustained engraftment at 1 year and 88.9% feasibility (95% CI, 70.8-97.7). The OS for the 27 patients was 92% (95% CI, 83-100) at 1, 2, and 3 years (Figure 1). This compares favorably with the Center for International Blood and Bone Marrow Transplant Research estimates of 1-year survival for upfront nonmyeloablative SAA transplants between July 1999 and June 2012. For patients aged ≤20 years (n = 1126), the 1-year survival was 79% (95% CI, 74-84) and in patients aged >20 years (n = 975) it was 68% (95% CI, 62-74).
Patient outcomes with haploidentical donor BMT with posttransplant cyclophosphamide. (A) OS based on the TBI dose. (B) Graft-failure–free (event-free) survival.
Patient outcomes with haploidentical donor BMT with posttransplant cyclophosphamide. (A) OS based on the TBI dose. (B) Graft-failure–free (event-free) survival.
The proportion of patients alive with engraftment at 1 year (graft failure–free survival was 89% [95% CI, 77-100]). Twenty-four patients had sustained >95% donor chimerism in both whole blood and CD3 compartments through 1 year. TBI dose was increased from 200 to 400 cGy to reduce graft failure after graft loss in 3 of the first 7 patients. All 20 consecutive patients who received 400 cGy are alive and well with >95% donor engraftment in whole blood and CD3 compartments. There were no withdrawals reported among the study patients.
Two (6%) deaths were reported after the transplant in patients who received 200 cGy TBI; 2 patients died of infection (CMV in an adult patient with secondary graft failure and EBV in a pediatric patient with primary graft failure); and a third patient had secondary graft failure after 200 cGy TBI but is now 100% chimeric using the identical conditioning platform and a second HLA-haploidentical (younger cousin after older parent) donor.
Twenty-six of 27 patients achieved neutrophil recovery by day 28. The median time to neutrophil recovery was 17 days (range, 14-88 days). The day-28 cumulative incidence of neutrophil recovery was 96% (95% CI, 87-100). The median time to platelet recovery was 25.5 days, with 90% transfusion independence by day 100. The day-100 incidence of platelet recovery was 88% (95% CI, 74-100) The median time to red blood cell recovery was 25.5 days, with 90% transfusion independence by day 60. All alive patients currently are in a CR, based on the noted hemogram metrics at the time of the last follow-up. Of the patients with engraftment, at 6 months 22 of 24 were in a CR, and at 12 months 23 of 24 met criteria for CR. The 1 patient with ongoing transfusion dependence at 1 year was the recipient of a product from a major ABO-incompatible donor but had complete donor engraftment with normal neutrophil and platelet counts. The patient was transfusion independent by year 2. The transfusion burden was a median of 10 red units (range, 3-172 red units) and 16.5 apheresis platelet products (range, 6-307 apheresis platelet products).
Eighteen (67%) individual patients experienced infections after transplant (Table 2). Of a total 39 infection events, 37 were grade 2 and 2 were grade 5. Both of the documented grade 5 infections occurred in the 2 patients who lost grafts and died of viral infection in that setting. The majority of documented infections were viral reactivation, documented via polymerase chain reaction, followed by bacterial infections. Four patients had documented fungal infections, of which 2 were in patients with graft failure; these patients did not pursue a second transplant. Eleven patients experienced CMV reactivation, with 6 of the 11 (55%) requiring therapy. One patient had documented EBV-associated posttransplant lymphoproliferative disease that developed after the secondary graft failure and died as a result. Of the entire patient cohort, 93% did not require any ventilator support during their BMT period; the only 2 patients who were intubated subsequently died. The adult patients treated on this trial were followed up daily at the ambulatory transplant clinic. Seventeen of 19 required admission for neutropenic fevers, but, for those admissions, the length of hospitalization was 4 days (range, 2-91 days). The pediatric patients received transplants in the inpatient setting, as per the practice at the institution. All patients could be discharged upon engraftment, with readmission in only 2 patients. Both the pediatric and adult patients who died were at the hospital at the time.
Infections
| . | Total, n (%) . |
|---|---|
| No. of patients who received transplant | 27 |
| No. of patients with infections | 18 (67) |
| No. of patients with infection reports | |
| 1 | 4 (15) |
| 2 | 9 (33) |
| 3 | 3 (11) |
| 4 | 2 (7) |
| 5 | 0 |
| ≥6 | 0 |
| Total infection events | 39 |
| Maximum severity based on the patient | |
| None | 0 |
| Grade 2 | 37 |
| Grade 5 | 2 |
| Infection type (no. of patients) | |
| Bacterial | 11 |
| Viral | 20 |
| Fungal | 4 |
| Incidence of CMV infection (no. of patients) | 11 |
| CMV infection requiring therapy | 6 |
| Incidence of EBV infection (no. of patients) | 2 |
| EBV infection requiring therapy | 1 |
| Incidence of PTLD (no. of patients) | 1 |
| . | Total, n (%) . |
|---|---|
| No. of patients who received transplant | 27 |
| No. of patients with infections | 18 (67) |
| No. of patients with infection reports | |
| 1 | 4 (15) |
| 2 | 9 (33) |
| 3 | 3 (11) |
| 4 | 2 (7) |
| 5 | 0 |
| ≥6 | 0 |
| Total infection events | 39 |
| Maximum severity based on the patient | |
| None | 0 |
| Grade 2 | 37 |
| Grade 5 | 2 |
| Infection type (no. of patients) | |
| Bacterial | 11 |
| Viral | 20 |
| Fungal | 4 |
| Incidence of CMV infection (no. of patients) | 11 |
| CMV infection requiring therapy | 6 |
| Incidence of EBV infection (no. of patients) | 2 |
| EBV infection requiring therapy | 1 |
| Incidence of PTLD (no. of patients) | 1 |
PTLD, posttransplant lymphoproliferative disease
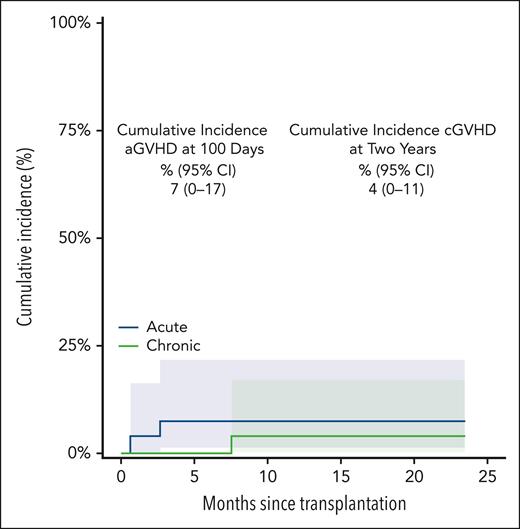
Rates of both acute and chronic GVHD were <10%, and no patient developed grade 3 or 4 acute GVHD or moderate/severe chronic GVHD. The cumulative incidence of grade 2 or 4 acute GVHD on day 100 was 7% (95% CI, not applicable [NA]-17), and that of chronic GVHD at 2 years was 4% (95% CI, NA-11). (Figure 2) Because all patients’ statuses were beyond 6 months of follow-up, immunosuppression was discontinued for all. The single patient with chronic GVHD noted at 8 months after BMT was off all treatment by month 11 after BMT. Formal immune reconstitution studies were not performed. No secondary malignancies have been observed. Fertility assessment has not been followed in a systematic way. Of the 25 living patients with engraftment, 24 currently had a Karnofsky performance status of >90% and, now, have returned to previous employment, or schooling.
Donor bone marrow grafts had a median total nucleated marrow cell count of 4.9 × 108/kg recipient IBW (range, 2.83 × 108-7.7 × 108/kg recipient IBW), a median CD34+ cell count of 5.8 × 106/kg recipient IBW (range, 2.59 × 106-10.7 × 106/kg recipient IBW), and a median CD3+ cell count of 5.1 ×107/kg recipient IBW (range, 2.8 × 107-8.2 × 107/kg recipient IBW).
Discussion
In a single-center study, we demonstrate an OS rate of 92% at 3 years using HLA-haploidentical BMT for pediatric and adult patients with treatment-naive SAA. The OS for those receiving 400 cGy of TBI was 100%. This likely represents a cure for these patients who did not have to undergo IST, a therapy whose avoidance may have significant merit, as already discussed. Furthermore, more than half of these patients had very severe aplastic anemia (ANC < 200 × 109/L), for whom the IST early mortality historically remains more than 10%.21 Longer-term follow-up for secondary malignancies and fertility is warranted for the development of other late sequelae. All patients are alive and have remained disease free since the augmentation to 400 cGy of TBI. In addition, this trial was able to enroll 37% of patients who did not self-identify their race/ethnicity as White, opening up this curative treatment to patients of many ethnic backgrounds. Eleven (40%) of the patients in the cohort were aged >40 years and 9 >50 years, suggesting this is not necessarily an approach only for the pediatric or adolescent populations.
The decision to pursue upfront BMT must consider alternative options for treatment-naive SAA. IST remains the standard of care for many, but this requires urgent reevaluation given improvements with BMT approaches such as those described here. There is little evidence for the superiority of IST with the safety of newer BMT platforms compared with older ones, but, currently, we lack prospective randomized data.27 Our data suggest that upfront allogeneic transplant should also be a consideration. The prognosis for patients with SAA treated with IST remains suboptimal, with relapse rates up to 40%, and lifetime evolution to MDS >10% or 15% in several series.6,28-30 More recently, eltrombopag has been shown to increase response rates in adult patients5 and possibly in children31 but does not seem to address the problem of relapse or secondary clonal disease. This represents many years of health care interactions and anxiety about the future risk to patients who likely still require BMT as salvage at some point. A long-term follow-up paper of a large European SAA cohort treated with horse ATG + cyclosporine demonstrated actuarial OS of 60% at 15 years with event-free survival of only 23% over the same time.21 The cumulative probability of being treated with transplant in that cohort at 15 years was between 14% and 22%.21 More recently, De Latour et al5 reported the outcomes of a phase 3 trial of the addition of eltrombopag to standard IST for untreated patients with SAA. In the triple therapy arm, nearly 100 patients had an overall response rate of 68% between 3 and 6 months, similar to previous results. Median follow-up was 24 months, with event-free survival of only 46% in the eltrombopag arm. This is a patient group who desperately requires meaningful therapies at the time of initial diagnosis to avoid complications of relapse or nonresponsiveness to frontline IST.
Often, the toxicity of BMT over IST is cited as a reason not to pursue BMT. However, here, in a wide range of patients, the treatment-related mortality when using 400 cGy TBI was 0%. BMT response and OS as described here compares favorably with the reported OS in both arms of the recent study for upfront triple drug IST,5 although no formal statistical comparisons can be performed. However, what must be emphasized over IST with this BMT platform is the rapidity of hematopoietic reconstitution, which can happen within a month for neutrophils and within 2 months for transfusion independence in the majority of patients. At 12 months, the CR rates to IST ranged from 33% to 52%,5 whereas here with BMT, response in patients with engraftment was >90%. The infectious complications in the BMT setting are more than that observed in IST paradigms but very manageable with rapid engraftment; overall, the infection rates are low. We expect rates of reactivation for CMV to perhaps lessen in the future with the implementation of newer agents for prophylaxis such as letermovir. The issue of active drugs for immunosuppression over longer periods is also complex. A recent, 4-year follow-up report of triple IST revealed a cumulative relapse rate of 39% in patients who were responding to cyclosporine maintenance (for at least 2 years) as well as clonal evolution of 15% in all treated patients at 4 years.30 With this BMT regimen, the PTCy for GVHD prophylaxis allows the majority of patients to come off tacrolimus by 6 months and all patients are off all immunosuppression by 1 year after BMT. The latter point also illustrates the more limited cost of a BMT approach. With an IST platform, eltrombopag adds substantial financial toxicity to the cost of standard IST as well as the needed 2 years of cyclosporine to avoid early relapse.4 When BMT represents a cure, has a shorter duration of total therapy, no greater toxicity, and costs the same as or less than IST, any benefit of IST is lost. Of note, in other retrospective series, haploidentical BMT outcomes have been similar to related donors in this regard.32 In fact, the 3 patients who lacked haploidentical donors were able to use this platform with unrelated matched donors. Longer-term risks such as the development of secondary malignancy or clonal evolution do require ongoing study. Historically, patients primarily develop hematologic malignancies after IST compared with solid tumor development after BMT. The latter is driven by both the presence of GVHD and perhaps the use of radiation in the conditioning regimen.33 The dose of radiation used here is less likely to cause these cancers in some series34,35; however, TBI in combination with reduced-intensity conditioning has been noted as a secondary cancer risk in sickle cell disease,36 so close follow-up is ongoing.
The therapeutic decision at the time of diagnosis of SAA is multifaceted. It is dependent upon physician expertise and comfort, patient preferences, and donor availability. This study illustrates a contemporary BMT approach that patients could have access to from early in their treatment course. Most patients with SAA without serious comorbidities, regardless of age and donor status, can now be considered as allogeneic BMT candidates based on the low rates of GVHD and transplant-related morbidity associated with PTCy. Historically, a reason cited for the upfront use of IST in older patients, regardless of matched donor availability, was increased rates of these toxicities.37 Patients could possibly expect lower toxicity, more rapid hematologic reconstitution, improved return to premorbid baseline functionality, and potentially less financial burden with BMT over IST. Here, the OS was 100% when 400 cGy of TBI was used. As patients share exactly 1 HLA-haploidentical type with each biological parent or child, half of their siblings, and half of their second-degree relatives (nieces, nephews, aunts, uncles, grandchildren, and grandparents),25,38,39 an eligible HLA-haploidentical donor can be identified in nearly all patients. Moreover, the problematic availability of matched unrelated donors for unrepresented minorities is addressed by this protocol and HLA-haploidentical BMT in general,40 allowing donors to be found in the 37% of nonWhite patients in our cohort. Limitations of the current study include a lack of available information on the transfusions before 6 weeks preconditioning, and that there was inconsistency in time to BMT from diagnosis, mainly attributable to referral patterns, not donor availability. We were able to move expeditiously in the majority of patients. Additionally, we only followed up patients for early events on trial, so data on fertility are lacking (anti-Mullerian hormone or pregnancies; anecdotes only) and still need longer follow-up for secondary malignancies. Additional follow-up will also show return to baseline lifestyles (that of before SAA diagnosis) more readily for patients who received BMT given they are likely cured, off immunosuppression, and without need for disease-related health care interactions. This study highlights the need for reevaluation of the role of BMT in upfront management of patients who do not have an available sibling donor, particularly in comparing this approach with the use of IST in a randomized controlled manner via an adequately powered prospective clinical trial.
Acknowledgments
The authors are grateful to the patients, their families, and the research staff for the ability to complete this study.
Support for this clinical trial was provided to A.E.D and Johns Hopkins University by Regenerative Medicine to Restore Normal Hematopoiesis in Aplastic Anemia, 2016-MSCRFE-2622 Maryland Stem Cell Research Fund (TEDCO). TEDCO served no direct role in the study design, or collection, analysis, or interpretation of the data, nor in the writing of the report.
Authorship
Contribution: A.E.D., R.A.B., and R.J.J. designed the study, treated the patients, analyzed the results, and wrote the manuscript; M.Z. performed the statistical analysis and reviewed the manuscript; and all other authors advised on the protocol, treated the patients at centers where the trial was open, analyzed the results, and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Amy E. DeZern, 1650 Orleans St, CRBI Room 3M87, Baltimore, MD 21287; e-mail: adezern1@jhmi.edu.
References
Author notes
Data are available on request from the corresponding author, Amy E. DeZern (adezern1@jhmi.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal