In this issue of Blood, Liu et al1 provide evidence that gasdermin D (GSDMD) expressed by neutrophils is not necessarily detrimental in sepsis, and in fact, GSDMD seems to protect the host from organ injury and death.
GSDMD initiates and drives pyroptosis, an inflammatory form of lytic cell death that is induced upon activation of inflammasomes by canonical (eg, caspase 1-dependent) or noncanonical (eg, caspase 11-driven) pathways.2 GSDMD controls the release of proinflammatory cytokines and also forms pores in the cell membrane, leading to cell rupture. Thus, pyroptosis controls the course of viral/bacterial infections by regulating the release of cytokines and removal of a potential replicative niche of a pathogen (within that leukocyte).2
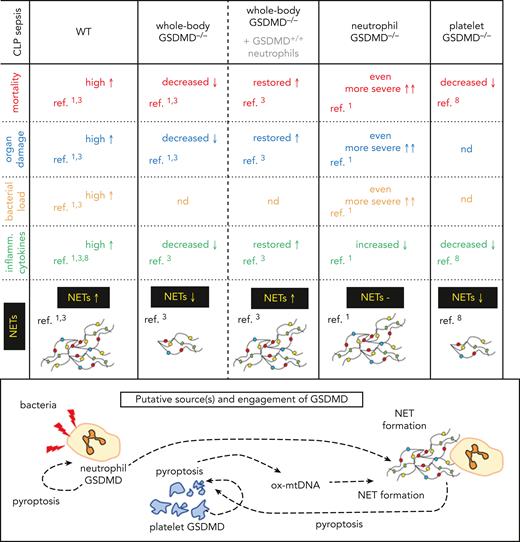
The current publication by Liu et al must be evaluated in light of another article published in Blood in 2021. In this earlier work, Silva et al also studied the role of GSDMD in sepsis,3 applying the same model of cecal ligation and puncture (CLP) but using whole-body GSDMD-deficient mice (GSDMD−/−) as the genetic model. In contrast, Liu et al utilized a neutrophil-specific conditional GSDMD knockout (KO) model (neutrophil-GSDMD−/−). Interestingly, these 2 studies reported contradictory findings (see figure). Liu et al observed an exacerbation of sepsis severity in mice when GSDMD was absent only in neutrophils, whereas when GSDMD was not produced systemically, sepsis was less severe.1,3 This concerned organ damage, mortality, proinflammatory cytokine storm, and neutrophil extracellular trap (NET) release (see figure).
Comparison of major findings on various genetic models of GSDMD deficiency on the course of CLP sepsis. Whole-body or neutrophil-only and platelet-only knockout (−/−) mice were studied. In some animals transfer of GSDMD-expressing neutrophils (+/+) was performed. −, none or unchanged parameter; ↑, increased; ↓, decreased; Inflamm., proinflammatory; ox-mtDNA, oxidized mitochondrial DNA; ref., reference; nd, not determined.
Comparison of major findings on various genetic models of GSDMD deficiency on the course of CLP sepsis. Whole-body or neutrophil-only and platelet-only knockout (−/−) mice were studied. In some animals transfer of GSDMD-expressing neutrophils (+/+) was performed. −, none or unchanged parameter; ↑, increased; ↓, decreased; Inflamm., proinflammatory; ox-mtDNA, oxidized mitochondrial DNA; ref., reference; nd, not determined.
Numerous studies reported previously that GSDMD is important for NET formation.4 Moreover, it has been shown that GSDMD can be activated by neutrophil proteases,4 although during CLP sepsis, caspase-11 has been demonstrated to be involved in NET formation.3 NETs consist of decondensed chromatin decorated with granular proteins, and these traps serve to immobilize/neutralize pathogens.5,6 However, when NETs are overproduced, or are not removed efficiently or in a timely fashion, they can cause collateral damage.7 The most striking finding of Liu et al was that GSDMD-deficient neutrophils were releasing NETs to the same extent as GSDMD-sufficient cells. Importantly, in another study, Su et al8 showed that during CLP sepsis, GSDMD is upregulated in platelets, important modulators of NET formation.5,6 These platelets undergo pyroptosis and release their contents, including oxidized mitochondrial DNA, which can trigger NET release.8 NETs in return further augment pyroptosis, forming a positive feedback loop between platelets and NETs, a process that was demonstrated using mice with GSDMD-deficient platelets (see figure).8 This observation potentially clarifies the discrepancy between findings of Liu et al and Silva et al with regards to NETs. Although NET release is driven by GSDMD, it may not be necessary that GSDMD is expressed by neutrophils themselves, indicating that neutrophils alone are not responsible for all bystander damage during sepsis.
Silva et al, aware of the potential limitation of their whole-body GSDMD-KO model, performed elegant experiments in which wild-type (WT) neutrophils were transferred to GSDMD-KO mice, and this restored the original, strongly inflammatory phenotype. It should be noted, as pointed out by Liu et al, that the process of neutrophil adoptive transfer itself can lead to inflammation. As such, to clearly dissect the role of neutrophil-GSDMD, parallel transfers should be performed using neutrophil GSDMD-deficient mice. In the light of the findings by Su et al,8 additional experiments could further assess the impact of transfer of GSDMD+/+ platelets into neutrophil GSDMD−/− and whole-body GSDMD−/− mice, as such an approach was shown previously to restore the capacity to cast NETs by neutrophils in a model of obesity-altered systemic endotoxemia.6
To support their findings, Silva et al also used disulfiram as a pharmacological agent to inhibit GSDMD. However, this drug can also affect other pathways, acting as an inhibitor of acetaldehyde dehydrogenase or P-glycoprotein and impacting the cellular localization of efflux transporter ATPases (eg, ATP7A).9 Previous studies have demonstrated that neutrophils of ATP7A mutant mice release more NETs ex vivo, potentially confounding the findings in animals treated with disulfiram.10
So, what is the role of GSDMD in sepsis? Overall, this molecule still appears to be responsible for neutrophil activation/NET formation (1); however, neutrophils of caspase-11−/− (upstream activator of GSDMD) mice still released some NETs.3 We have learned that the source of GSDMD required for NET release might be nonneutrophilic (2), but it remains to be determined if this observation represents the canonical NET pathway or only acts as a compensatory pathway when neutrophilic GSDMD is unavailable. Moreover, (3) GSDMD-related bystander injury could be attributed to other leukocytes (eg, when Silva et al transferred GSDMD+/+ monocytes into the whole-body GSDMD KOs, elevated levels of proinflammatory cytokines were detected) and platelets. Finally, in relation to GSDMD and pyroptosis, neutrophils appear not to be detrimental (or at least, not exclusively) in sepsis as their absence increased the severity of CLP sepsis. One possible explanation for this observation is that whereas some or numerous WT neutrophils die by pyroptosis upon GSDMD activation during sepsis, this naturally eliminates the neutrophilic niche for bacteria replication, leading to exaggerated inflammation. Although in the past neutrophils have not been classically associated with intracellular bacteria, it is now acknowledged that they might serve as temporary reservoirs for pathogens, including microbes that have been phagocytized. Considering that some neutrophils perform reverse migration and return to the blood, cells that fail to die through pyroptosis might represent vectors that lead to the spread of infection systemically.
Perhaps the most important takeaway from the previously mentioned studies is the demonstration of the value of cell-specific knockout models. The use of a neutrophil-specific deletion, and comparing these findings to those previously reported for whole-body KO animals, offers better understanding of the complex biology involved in sepsis, providing a more detailed, and more accurate, map of involved mechanisms. Undoubtably, GSDMD significantly contributes to the pathology of sepsis; however, its role in pyroptosis, NET release, and sepsis is nuanced, and further studies are required to fully elucidate its role.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal