Key Points
PP2A activators enhance the efficacy of venetoclax and venetoclax-azacitidine combination in primary samples and preclinical models of AML.
PP2A-B56α holoenzyme drives this synergy by modulating BCL2 and MCL1, and PPP2R5A expression is a potential biomarker to predict responses.
Abstract
Venetoclax combination therapies are becoming the standard of care in acute myeloid leukemia (AML). However, the therapeutic benefit of these drugs in older/unfit patients is limited to only a few months, highlighting the need for more effective therapies. Protein phosphatase 2A (PP2A) is a tumor suppressor phosphatase with pleiotropic functions that becomes inactivated in ∼70% of AML cases. PP2A promotes cancer cell death by modulating the phosphorylation state in a variety of proteins along the mitochondrial apoptotic pathway. We therefore hypothesized that pharmacological PP2A reactivation could increase BCL2 dependency in AML cells and, thus, potentiate venetoclax–induced cell death. Here, by using 3 structurally distinct PP2A-activating drugs, we show that PP2A reactivation synergistically enhances venetoclax activity in AML cell lines, primary cells, and xenograft models. Through the use of gene editing tools and pharmacological approaches, we demonstrate that the observed therapeutic synergy relies on PP2A complexes containing the B56α regulatory subunit, of which expression dictates response to the combination therapy. Mechanistically, PP2A reactivation enhances venetoclax-driven apoptosis through simultaneous inhibition of antiapoptotic BCL2 and extracellular signal-regulated kinase signaling, with the latter decreasing MCL1 protein stability. Finally, PP2A targeting increases the efficacy of the clinically approved venetoclax and azacitidine combination in vitro, in primary cells, and in an AML patient-derived xenograft model. These preclinical results provide a scientific rationale for testing PP2A-activating drugs with venetoclax combinations in AML.
Introduction
Acute myeloid leukemia (AML) is a disease of older persons that has a dismal outcome. Intensive therapeutic regimens result in 5-year survival rates of 30% to 35% for patients aged <60 years, and 5% to 15% for older patients.1,2 Patients unfit for intensive chemotherapy exhibit a median survival of 5 to 10 months.1 However, the therapeutic landscape of AML is changing with the appearance of new targeted drugs2-4 such as BH3 mimetics that inhibit prosurvival BCL2 family proteins.5 The BCL2-specific inhibitor venetoclax has already been approved by the US Food and Drug Administration for the treatment of different leukemias, while other drugs targeting MCL1 and BCL-XL, such as S-638456 or A-1331852,7 undergo clinical evaluation. In AML, the activity of venetoclax as a single agent has been modest;8 nevertheless, combinations with either hypomethylating agents or low-dose chemotherapy demonstrated impressive clinical benefit in older/unfit patients,9,10 and have been approved as frontline therapies.11 However, despite promising results of phase 3 clinical trials, longer follow-up has shown that venetoclax combinations extend median overall survival (OS) by only 4 to 5 months.12,13 Durability of response after venetoclax combination therapies is compromised by both primary and adaptive resistance, with high expression levels of MCL1 as one of the key mechanisms of poor response.5,8,14 Furthermore, management of patients who are initially refractory to venetoclax combinations or who relapse remains challenging.15 These data highlight the critical need for more effective venetoclax combination therapies.
Protein phosphatase 2A (PP2A), one of the main serine (Ser)/threonine (Thr) phosphatases, is a tumor suppressor that regulates essential functions by counteracting most kinase-driven intracellular signaling pathways.16,17 Such wide-ranging substrate specificity is achieved by the formation of distinct heterotrimeric holoenzymes, each containing a catalytic (C), a scaffold (A), and a regulatory (B) subunit. Regulatory B subunits are critical because they dictate substrate specificity and localization of PP2A complexes. There are >23 isoforms belonging to 4 families: B55, B56, PR72/130, and STRN.16,17 B56- and B55-containing PP2A complexes direct most of the tumor-suppressive activity of PP2A.16,17 PP2A is inactivated across a broad range of hematological and solid tumors.18 In AML, we, and others, have reported inactivation of PP2A in ∼70% of cases.19 Besides, we found that pharmacological restoration of PP2A activity by FTY720 and our recently developed CM-1231,20 a nonphosphorylable analog of FTY720, have potent antitumor effects in vitro and in vivo.19-22 Novel PP2A-activating compounds, such as DT-061, selectively increase the activity of specific tumor-suppressive PP2A heterotrimers.23
Given that PP2A promotes cancer cell apoptosis by affecting the phosphorylation state of different proteins in the mitochondrial apoptotic pathway,17,24,25 we hypothesized that PP2A reactivation might have therapeutic value in AML by triggering mitochondrial apoptosis. Here, by integrating studies in AML cell lines, primary cells, and distinct xenograft models, we show that PP2A-activating drugs sensitize AML cells to venetoclax-mediated cytotoxicity through dual inactivation of BCL2 and MCL1. Using 3 different structural PP2A activators and CRISPR–CRISPR-associated protein 9 (CRISPR-Cas9) genetic approaches, we decipher the synergistic mechanism of this novel combination, with the PP2A-B56α holoenzyme as the critical regulator of this therapeutic response. Of importance, we demonstrate that the antileukemic effect of clinically approved venetoclax-azacitidine combination is synergistically enhanced by PP2A reactivation in vitro, in primary AML blasts, and in vivo. Collectively, our findings provide preclinical evidence for combining PP2A-activating drugs with venetoclax or venetoclax-azacitidine as a new therapeutic strategy for the treatment of AML.
Methods
Human cell lines and compounds
PEER, CCRF-CEM, Jurkat, MOLT-4, and P12-ICHIKAWA cells were kindly provided by Jan Cools (Leuven, Belgium). Karpas-422 cells were previously described.26 All other cell lines were obtained from DSMZ (supplemental Table 1, available on the Blood website). Cell lines were maintained in appropriate culture medium supplemented with fetal bovine serum, penicillin G (100 U/mL), and streptomycin (0.1 mg/mL), and grown at 37°C in a 5% CO2 atmosphere. Cultured cells were regularly tested negative for Mycoplasma. Cells were treated with FTY720, venetoclax, A-1331852, azacitidine, DT-061 (Selleckchem), S-63845 (ChemieTek), CM-1231,20 z-VAD-FMK (ApexBio), cycloheximide (Sigma), and trametinib (CymitQuimica).
Primary AML cells and normal bone marrow CD34+ cells
Primary AML cells were purified from 26 patients with AML at diagnosis (supplemental Table 2). Patients gave written informed consent. Samples were collected in accordance with the Declaration of Helsinki, with approval of the Ethics Committee Investigación Clínica de Navarra (PI2018/32). Ammonium chloride potassium buffer was used to lyse red blood cells (10 mL ammonium chloride potassium buffer per 1 mL of sample, 15 minutes at 4°C). Cells were cultured in StemSpan SFEM II medium supplemented with StemSpan-CD34+-expansion supplement, UM729-pyrimido-indole derivative (StemSpan Leukemic Cell Culture Kit, STEMCELL Technologies), penicillin G (100 U/mL), and streptomycin (0.1 mg/mL) following the manufacturer’s instructions. Normal human bone marrow–derived CD34+ cells were obtained from STEMCELL Technologies.
Cell viability and drug combination assays
Human cell lines (25 000-35 000 cells per well) or AML primary cells (20 000 cells per well) were plated in 96-well plates, and compounds were added. Growth inhibition was determined at multiple concentrations of PP2A-activating drugs in combination with multiple concentrations of BH3 mimetics and/or azacitidine. Cell viability was determined as a readout using Cell Titer96 Aqueous One-Solution Cell Proliferation assay after 48 hours (cell lines) or 60 hours (primary AML cells) of drug treatment. For 2-drug combination experiments, combination index (CI) values were calculated according to the methods of Chou, using the CompuSyn software (Combosyn).27 CI values were used to determine whether drug combinations were synergistic (CI < 1), additive (CI = 1), or antagonistic (CI > 1). For 3-drug combination analyses, zero interaction potency (ZIP) synergism scores were calculated using the SynergyFinder Plus webtool.28,29 A combined treatment was considered synergistic when the zero interaction potency synergism score was >10.
Murine models
AML subcutaneous model
HL-60-Cas9 clone 1 (B56α-wild-type, 5 × 105) or clone 2 cells (B56α-inactivation, 5 × 105) in phosphate-buffered saline and matrigel (Corning) (1:1) were injected subcutaneously into the flanks of 8- to 10-week-old female NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. Once tumor volumes reached 100 to 150 mm3, mice were randomized and given vehicle (10% N,N-dimethylacetamide [DMA]), 10% Kolliphor HS15, 80% water), FTY720 (7.5 mg/kg in water), venetoclax (30 mg/kg in 10% DMA, 10% Kolliphor HS15, 80% water), or the combination of FTY720 and venetoclax by oral gavage (Figure 4D). Treatment was administered for 2 weeks. Tumor volumes were assessed by caliper measurement (L × W2/2).
AML PDX model
Primary AML cells (AML-2 cells) were injected intravenously into the tail vein of 8- to 10-week-old female NSG mice (per mouse: 2 × 106 cells in 200 μL of phosphate-buffered saline). Animals were randomized to 3 treatment groups: (1) vehicle (10% DMA, 10% Kolliphor-HS15, 80% H2O), (2) venetoclax (100 mg/kg) and azacitidine (4 mg/kg), or (3) FTY720 (7.5 mg/kg), venetoclax (100 mg/kg), and azacitidine (4 mg/kg). Treatment was started 5 days postinjection and continued for 8 weeks (Figure 5G). In vivo experiments were approved by the University of Michigan Institutional Animal Care and Use Committee, and the University of Navarra Ethics Committee for Animal Experimentation.
Statistical analyses
Data were analyzed using GraphPad Prism version 7 (GraphPad Software) for parametric statistical tests (unpaired t test, analysis of variance) when data followed a normal distribution (Shapiro-Wilk, Kolmogorov-Smirnov) and equal variances (Levene test), or adequate nonparametric statistical tests (Mann-Whitney U, Kruskal-Wallis, Mantel-Cox). Regarding in vitro experiments, data shown represent the outcomes of 3 to 4 independent biological replicates, which were run in 2 to 3 technical replicates (unless otherwise specified). In vivo experiments were performed as specified in the corresponding figures/figure legends.
Results
Combination of PP2A-activating drugs and venetoclax induces potent and synergistic antileukemic effects in AML cells
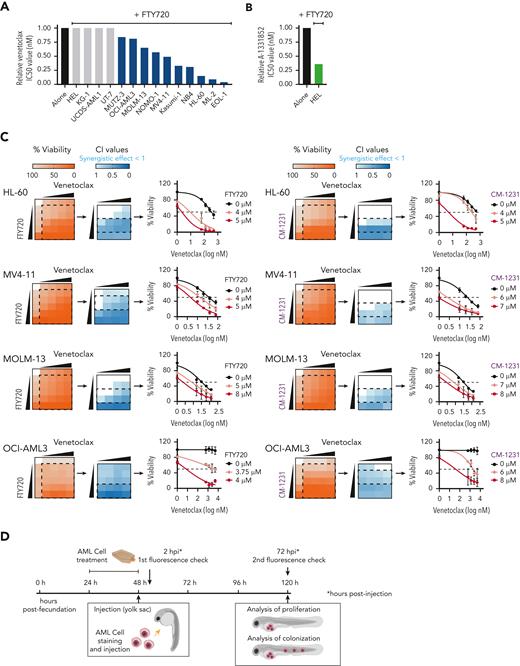
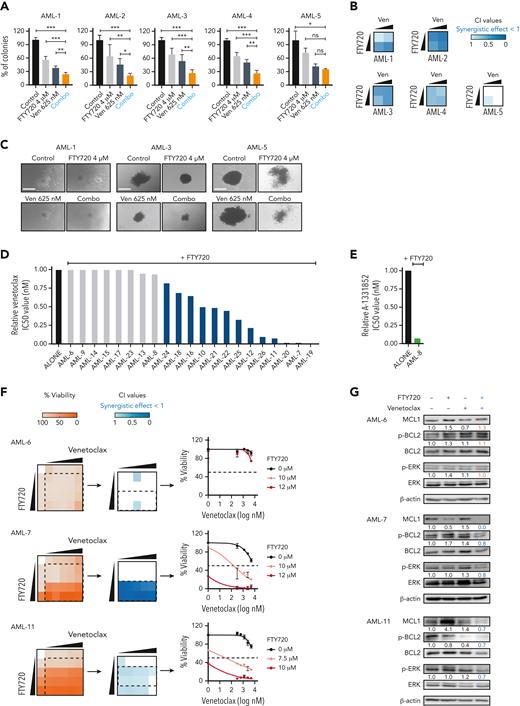
To investigate whether the PP2A-activating compound FTY720 enhances venetoclax activity, we first evaluated the cytotoxic effect of both drugs in 14 human AML cell lines (supplemental Table 1). Cell viability assays after FTY720, venetoclax, or the combined treatment at different concentrations showed that FTY720 plus venetoclax resulted in synergistic antileukemic effects in 10 of the 14 AML cell lines tested, including both venetoclax-sensitive and -resistant cells (Figure 1A,C; supplemental Table 1). HEL cells, with low BCL2 and high BCL-XL expression were resistant to the combined treatment; however, the combination FTY720 plus A-1331852 (BCL-XL inhibitor) was synergistic in this model (Figure 1A-B; supplemental Figure 1A-B). In addition, potent antileukemic effects were observed upon combination of the nonphosphorylable FTY720 analog CM-1231 (which has the same mechanism of action as FTY720)20 and BH3 mimetic drugs (Figure 1C; supplemental Figure 1B).
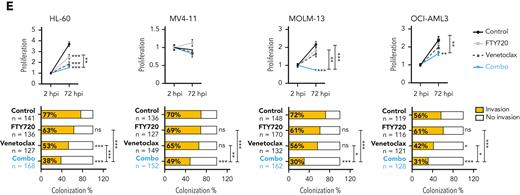
PP2A activators in combination with the BCL2 inhibitor venetoclax exhibits synergistic antileukemic activity in AML cell lines in vitro and in vivo. Bar graphs show the reduction of venetoclax IC50 values (A) or A-1331852 (BCL-XL inhibitor) IC50 values (B) when combined with FTY720 in a panel of 14 AML cell lines (48 hours of treatment). (C) Heatmap representations of viability percentages and CI values along with cell viability curves of combined PP2A-activating drugs FTY720 or CM-1231 with venetoclax at increasing concentrations (48 hours of treatment). Dashed rectangles highlight data represented in the next graph. (D) After 24 hours of treatment, stained AML cells were injected into the yolk sac of dechorionated Casper zebrafish embryos (48 hours postfecundation). Proliferation and colonization analyses were performed at 72 hours postinjection (hpi). (E) Quantification of cell proliferation and colonization potential of AML cell lines injected into zebrafish embryos after drug exposure. All experiments were done in triplicate; the number of embryos used is indicated as “n.” Proliferation fold change (line graphs) was calculated as the difference between fluorescent readouts at 2 and 72 hpi. Statistical significance was determined by two-way ANOVA, followed by Tukey post hoc test for multiple comparisons. For analysis of colonization potential (bar graphs), zebrafish embryos were categorized into invasion or noninvasion groups. Data were analyzed by χ2 tests; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. ANOVA, analysis of variance; ns, not statistically different.
PP2A activators in combination with the BCL2 inhibitor venetoclax exhibits synergistic antileukemic activity in AML cell lines in vitro and in vivo. Bar graphs show the reduction of venetoclax IC50 values (A) or A-1331852 (BCL-XL inhibitor) IC50 values (B) when combined with FTY720 in a panel of 14 AML cell lines (48 hours of treatment). (C) Heatmap representations of viability percentages and CI values along with cell viability curves of combined PP2A-activating drugs FTY720 or CM-1231 with venetoclax at increasing concentrations (48 hours of treatment). Dashed rectangles highlight data represented in the next graph. (D) After 24 hours of treatment, stained AML cells were injected into the yolk sac of dechorionated Casper zebrafish embryos (48 hours postfecundation). Proliferation and colonization analyses were performed at 72 hours postinjection (hpi). (E) Quantification of cell proliferation and colonization potential of AML cell lines injected into zebrafish embryos after drug exposure. All experiments were done in triplicate; the number of embryos used is indicated as “n.” Proliferation fold change (line graphs) was calculated as the difference between fluorescent readouts at 2 and 72 hpi. Statistical significance was determined by two-way ANOVA, followed by Tukey post hoc test for multiple comparisons. For analysis of colonization potential (bar graphs), zebrafish embryos were categorized into invasion or noninvasion groups. Data were analyzed by χ2 tests; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. ANOVA, analysis of variance; ns, not statistically different.
We also evaluated the effect of these drugs in human AML xenografts in zebrafish embryos (Figure 1D), because they allow noninvasive imaging of tumor growth and the screening of a large number of individuals for treatment response.30 As cellular models, we chose 4 well-characterized AML cell lines with different genetic/cytogenetic backgrounds, HL-60, MV4-11, MOLM-13, and OCI-AML3. Compared with the control and single-therapy groups, treatment with FTY720 plus venetoclax synergistically reduced cell proliferation and colonization in all tested AML zebrafish models (Figure 1E; supplemental Figure 1C). Together, our results demonstrate that the combination of FTY720 and venetoclax significantly affects the leukemic potential of AML cell lines.
PP2A activation and venetoclax treatment synergize to induce caspase-dependent apoptosis through modulation of BCL2 and MCL1
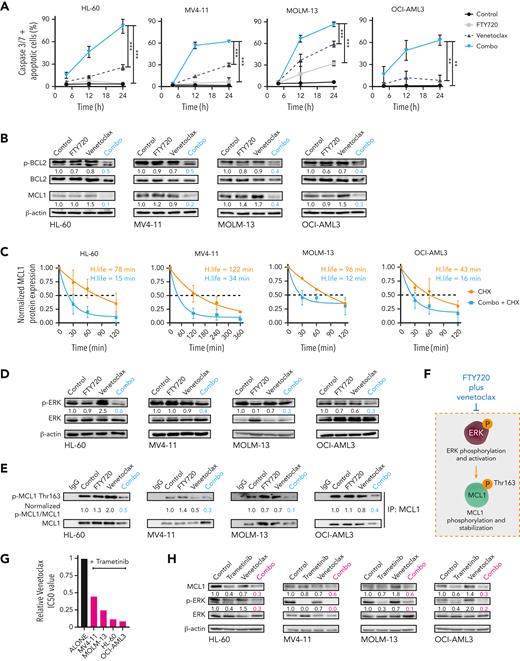
To investigate how FTY720 synergizes with venetoclax, time-course analyses of annexin V/propidium iodide staining and caspase-3 and -7 activation were carried out in selected AML cell lines. For these and subsequent experiments, both drugs were used alone or in combination at concentrations below their corresponding 50% inhibitory concentrations (IC50s). Combination therapy increased the number of apoptotic cells, compared with single treatments (supplemental Figure 2A), and induced a synergistic time-dependent activation of caspase-3 and -7 (Figure 2A). Western blot analysis confirmed caspase-3 and PARP cleavage upon this drug combination (supplemental Figure 2B). Moreover, FTY720 plus venetoclax significantly reduced the mitochondrial membrane potential, and z-VAD-FMK reversed the synergistic responses (supplemental Figure 2C-D). Therefore, combined FTY720 and venetoclax treatment induces synergistic caspase-dependent proapoptotic activity in AML cell lines.
The combination of FTY720 and venetoclax promotes caspase-dependent apoptosis through modulation of p-BCL2 and ERK-dependent MCL1 degradation. (A) Time-dependent caspase-3 and -7 activation plus apoptotic cells in AML cell lines after cell treatment. Statistical significance was determined by one-way ANOVA and Tukey post hoc test analysis (data at 24 hours); ∗∗P < .01, ∗∗∗P < .001. (B) Western blot analysis shows decreased Ser70p-BCL2 and MCL1 protein expression levels after 24 hours of treatment with FTY720 and venetoclax in combination in AML cell lines. (C) Half-life of normalized MCL1 protein levels as a function of time after adding CHX or CHX plus FTY720 and venetoclax. β-actin was used as loading control for normalization of MCL1 protein levels, which were expressed as fold change of the control (assigned a value of 1). Analyses were performed by using 1-phase decay equations. (D) Levels of Thr202/Tyr204p-ERK and ERK protein expression were significantly decreased in treated AML cell lines (24 hours of treatment). (E) Western blot analyses after MCL1 protein immunoprecipitation show that Thr163p-MCL1 was significantly reduced in AML cell lines upon treatment (24 hours). (F) Schematic representation showing that FTY720 plus venetoclax negatively regulates ERK-dependent stabilization of MCL1. (G) Bar graphs show reduction of venetoclax IC50 values when combined with trametinib in AML cell lines (48 hours of treatment). (H) Western blot analyses show significantly decreased MCL1 and Thr202/Tyr204p-ERK after 24 hours of treatment with trametinib and venetoclax combination. ANOVA, analysis of variance; CHX, cycloheximide.
The combination of FTY720 and venetoclax promotes caspase-dependent apoptosis through modulation of p-BCL2 and ERK-dependent MCL1 degradation. (A) Time-dependent caspase-3 and -7 activation plus apoptotic cells in AML cell lines after cell treatment. Statistical significance was determined by one-way ANOVA and Tukey post hoc test analysis (data at 24 hours); ∗∗P < .01, ∗∗∗P < .001. (B) Western blot analysis shows decreased Ser70p-BCL2 and MCL1 protein expression levels after 24 hours of treatment with FTY720 and venetoclax in combination in AML cell lines. (C) Half-life of normalized MCL1 protein levels as a function of time after adding CHX or CHX plus FTY720 and venetoclax. β-actin was used as loading control for normalization of MCL1 protein levels, which were expressed as fold change of the control (assigned a value of 1). Analyses were performed by using 1-phase decay equations. (D) Levels of Thr202/Tyr204p-ERK and ERK protein expression were significantly decreased in treated AML cell lines (24 hours of treatment). (E) Western blot analyses after MCL1 protein immunoprecipitation show that Thr163p-MCL1 was significantly reduced in AML cell lines upon treatment (24 hours). (F) Schematic representation showing that FTY720 plus venetoclax negatively regulates ERK-dependent stabilization of MCL1. (G) Bar graphs show reduction of venetoclax IC50 values when combined with trametinib in AML cell lines (48 hours of treatment). (H) Western blot analyses show significantly decreased MCL1 and Thr202/Tyr204p-ERK after 24 hours of treatment with trametinib and venetoclax combination. ANOVA, analysis of variance; CHX, cycloheximide.
Next, changes in the expression of proapoptotic and antiapoptotic proteins of the BCL2 family were measured upon drug treatment. No consistent differences in expression of BIM, BAD, NOXA, and PUMA were observed (supplemental Figure 2E). A marked reduction in MCL1 protein levels was detected upon addition of venetoclax with FTY720 treatment (Figure 2B). Literature has suggested a role for PP2A in the regulation of BCL2, thus, we checked for changes in BCL2 phosphorylation at Ser70.31 FTY720 and venetoclax treatment synergistically decreased BCL2 phosphorylation at this residue (Figure 2B). Similar results were obtained upon combining CM-1231 with venetoclax (supplemental Figure 2F), confirming that the observed phenotype relies on PP2A reactivation. When FTY720 and CM-1231 were used as single agents at high concentrations, changes in MCL1 and p-BCL2 protein levels were also observed, whereas venetoclax had little effect on the expression of these proteins (supplemental Figure 3A). This suggests that PP2A activation is responsible for MCL1 and p-BCL2 modulation, and that these effects are significantly potentiated by venetoclax.
Ectopic expression of MCL1 rendered cells more resistant to FTY720 plus venetoclax combination (supplemental Figure 3B-C), indicating that decreased MCL1 protein expression is key to the observed synergistic cytotoxicity. MCL1 messenger RNA (mRNA) expression remained unchanged upon drug treatment (supplemental Figure 3D), suggesting the involvement of posttranscriptional changes in the observed reduction of MCL1 protein expression. Accordingly, treatment with cycloheximide in the presence or absence of the combination therapy showed that the half-life of MCL1 protein was further reduced by cotreatment with FTY720 and venetoclax (Figure 2C). Given that extracellular signal-regulated kinase (ERK)-mediated phosphorylation of MCL1 at Thr163 retards MCL1 degradation,32 and that ERK is a target of PP2A,33 we hypothesized that PP2A activation could modulate ERK-driven MCL1 protein stability in AML cells. FTY720 in combination with venetoclax resulted in a significant reduction in ERK phosphorylation at Thr202/tyrosine 204 (Tyr204), and of MCL1 at Thr163 (Figure 2D-F). To evaluate whether ERK inactivation (ie, dephosphorylation) by FTY720-venetoclax combination therapy is important for efficacy, AML cells were treated with combined trametinib (MEK-ERK inhibitor) and venetoclax. As FTY720-venetoclax, this combination had antileukemic effects and showed decreased MCL1 protein levels (Figure 2G-H). In addition, levels of MCL1 and p-ERK were also reduced upon treating HEL cells with FTY720 and A-1331852, suggesting that FTY720 and BH3 mimetics synergize through ERK-driven MCL1 modulation (supplemental Figure 3E). Immunoprecipitation studies revealed that the combination of FTY720 and venetoclax impaired the interaction of both BCL2 and MCL1 with the proapoptotic protein BIM,34 thus enabling BIM release resulting in cell death (supplemental Figure 3F). Overall, our data show that FTY720 and venetoclax synergize through ERK-driven increased MCL1 protein turnover and BCL2 inactivation, triggering BIM release resulting in mitochondrial apoptosis.
FTY720 and venetoclax efficiently target primary AML cells from patients at high risk, ex vivo
Next, the antileukemic activity of FTY720 and venetoclax was analyzed in 5 primary AML samples (supplemental Table 2). Colony-forming unit (CFU) assays revealed that the response to this combined treatment (considering both the percentage and size of colonies) was synergistic in 4 of 5 samples (Figure 3A-C). To extend these results, leukemic cells from an additional 21 patients with AML were collected at diagnosis. For each sample, sensitivity to different BH3 mimetics was analyzed, as well as the protein expression levels of BCL2, MCL1, BCL-XL, SET, and PP2Ac (supplemental Figure 4A-B). Combination treatment with FTY720 plus venetoclax induced synergistic changes in cell viability in 13 of the 21 primary AML samples (Figure 3D,F). Sample AML-8, with high expression of BCL-XL, displayed synergistic effects upon exposure to FTY720 plus A-1331852 (Figure 3E; supplemental Figure 4C). Overall, our results show that 18 of 26 (69%) primary samples responded to FTY720 plus BH3 mimetics treatments. Notably, 14 of these 18 responders (78%) were classified as high risk according to the European LeukemiaNet 2022 guidelines.35 In addition, FTY720 plus BH3 mimetics synergistic responses were observed across different AML mutation subgroups (supplemental Figure 4D; supplemental Table 2).35,36 Combination therapy had minimal effects on normal human CD34+ hematopoietic stem/progenitor cells (supplemental Figure 4E).
Concomitant PP2A activation and BCL2 inhibition significantly decreases the clonogenic potential and proliferation of primary AML cells ex vivo. (A) Bar graphs show the percentage of colonies grown after exposure to different treatments. Data were analyzed by one-way ANOVA and Tukey post hoc test analyses; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. (B) Heatmaps show the CI values of FTY720 plus venetoclax in 5 primary AML samples. (C) Representative images of colonies grown upon treatment exposure. Scale bars: 400 μM. Bar graphs show reduction of venetoclax (D) or A-1331852 (BCL-XL inhibitor) (E) IC50 values upon combination with FTY720 in 21 distinct primary AML samples at diagnosis. (F) Heatmap representations of viability percentages and CI values, along with cell viability curves of FTY720 combined with venetoclax at increasing concentrations in cells from patients with AML (AML-6, AML-7, and AML-11) (60 hours of treatment). (G) After combo treatment (36 hours), MCL1, Ser70p-BCL2, and Thr202/Tyr204p-ERK levels were significant decreased in therapy-responsive samples (AML-7 and AML-11). ANOVA, analysis of variance; ns, not statistically different.
Concomitant PP2A activation and BCL2 inhibition significantly decreases the clonogenic potential and proliferation of primary AML cells ex vivo. (A) Bar graphs show the percentage of colonies grown after exposure to different treatments. Data were analyzed by one-way ANOVA and Tukey post hoc test analyses; ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. (B) Heatmaps show the CI values of FTY720 plus venetoclax in 5 primary AML samples. (C) Representative images of colonies grown upon treatment exposure. Scale bars: 400 μM. Bar graphs show reduction of venetoclax (D) or A-1331852 (BCL-XL inhibitor) (E) IC50 values upon combination with FTY720 in 21 distinct primary AML samples at diagnosis. (F) Heatmap representations of viability percentages and CI values, along with cell viability curves of FTY720 combined with venetoclax at increasing concentrations in cells from patients with AML (AML-6, AML-7, and AML-11) (60 hours of treatment). (G) After combo treatment (36 hours), MCL1, Ser70p-BCL2, and Thr202/Tyr204p-ERK levels were significant decreased in therapy-responsive samples (AML-7 and AML-11). ANOVA, analysis of variance; ns, not statistically different.
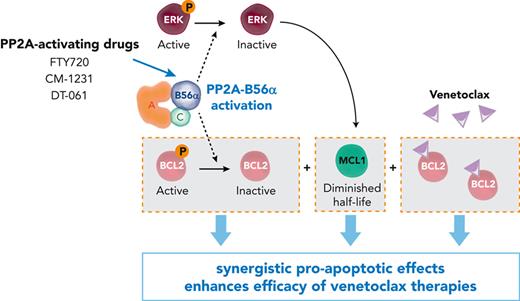
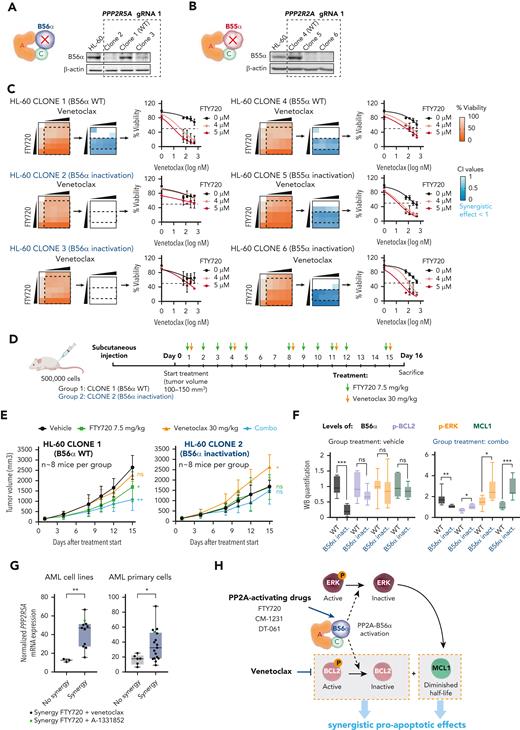
The PP2A regulatory subunit B56α is the master regulator of the observed treatment synergy in vitro and in vivo. Schematic representation and western blot analysis of B56α (A) and B55α (B) in HL-60 parental cells, wild-type clones (WT, 1 and 4), and clones with CRISPR-Cas9 inactivation of PPP2R5A (2 and 3) and PPP2R2A (5 and 6) genes. (C) Viability percentages, CI values, and cell viability curves show that loss of B56α rescues the synergistic treatment effect. (D) NSG mice were injected with HL-60 clone 1 (WT) or clone 2 (B56α inactivation) cells. When tumors reached 100 to 150 mm3, FTY720 and venetoclax were administrated by oral gavage. (E) Time-dependent tumor volume growth upon treatment of injected NSG mice (n = 8 mice per group). Data were analyzed by two-way ANOVA and Tukey post hoc test. (F) Western blot analysis of B56α, Ser70p-BCL2, Thr202/Tyr204p-ERK, and MCL1 in tumors isolated from mice after 15 days of treatment. Two-tailed unpaired t test or Mann-Whitney U test was used for statistical analyses. (G) Boxplot graphs show PPP2R5A mRNA expression in AML cell lines (left, n = 14) and AML primary cells (right, n = 23; samples with good mRNA quality were assessed by quantitative reverse transcription polymerase chain reaction). Both cell lines and AML primary blasts sensitive to the combined therapy have significantly higher PPP2R5A mRNA levels. Data were analyzed by two-tailed Mann-Whitney U test (cell lines) or unpaired t test (AML primary blasts). (H) Schematic illustration of the molecular mechanism underlying the synergistic effect between PP2A-activating drugs and venetoclax. In panels E-G: ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. ANOVA, analysis of variance; ns, not statistically different.
The PP2A regulatory subunit B56α is the master regulator of the observed treatment synergy in vitro and in vivo. Schematic representation and western blot analysis of B56α (A) and B55α (B) in HL-60 parental cells, wild-type clones (WT, 1 and 4), and clones with CRISPR-Cas9 inactivation of PPP2R5A (2 and 3) and PPP2R2A (5 and 6) genes. (C) Viability percentages, CI values, and cell viability curves show that loss of B56α rescues the synergistic treatment effect. (D) NSG mice were injected with HL-60 clone 1 (WT) or clone 2 (B56α inactivation) cells. When tumors reached 100 to 150 mm3, FTY720 and venetoclax were administrated by oral gavage. (E) Time-dependent tumor volume growth upon treatment of injected NSG mice (n = 8 mice per group). Data were analyzed by two-way ANOVA and Tukey post hoc test. (F) Western blot analysis of B56α, Ser70p-BCL2, Thr202/Tyr204p-ERK, and MCL1 in tumors isolated from mice after 15 days of treatment. Two-tailed unpaired t test or Mann-Whitney U test was used for statistical analyses. (G) Boxplot graphs show PPP2R5A mRNA expression in AML cell lines (left, n = 14) and AML primary cells (right, n = 23; samples with good mRNA quality were assessed by quantitative reverse transcription polymerase chain reaction). Both cell lines and AML primary blasts sensitive to the combined therapy have significantly higher PPP2R5A mRNA levels. Data were analyzed by two-tailed Mann-Whitney U test (cell lines) or unpaired t test (AML primary blasts). (H) Schematic illustration of the molecular mechanism underlying the synergistic effect between PP2A-activating drugs and venetoclax. In panels E-G: ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. ANOVA, analysis of variance; ns, not statistically different.
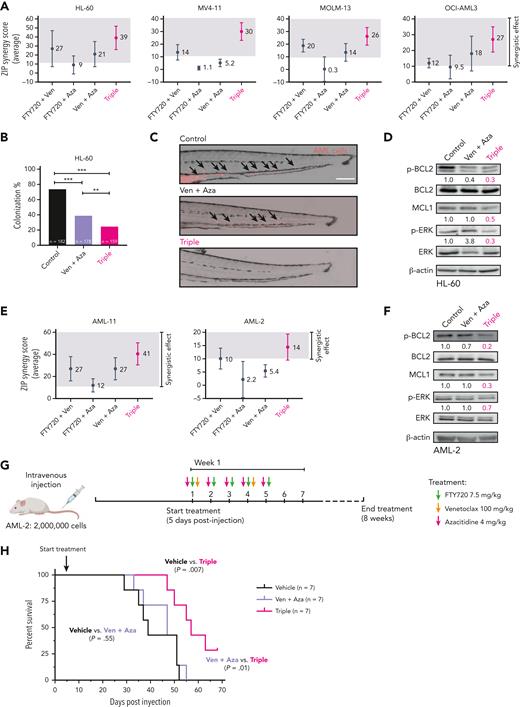
PP2A activation further improves the effectiveness of venetoclax plus azacitidine therapy in AML. (A) Multidrug synergy graphs show the average ZIP synergy score for double and triple combinations between FTY720, venetoclax, and azacitidine. Analyses were performed with the SynergyFinder Plus webtool. Synergy scores >10 (gray boxes) were considered synergistic. (B) Colonization potential of HL-60 cells in zebrafish xenograft models upon treatment with control, venetoclax plus azacitidine, or the triple therapy. Experiments were done in triplicate; number of embryos used is indicated as “n.” Data were analyzed by χ2 tests; ∗∗P < .01, ∗∗∗P < .001. (C) Representative images of HL-60 invading cells throughout the tail of zebrafish xenograft embryos at 72 hpi upon treatment with control, venetoclax plus azacitidine, or the triple therapy. AML cells were tracked as red-emitting fluorescent cells. Arrows show migrating HL-60 cells. Scale bar: 0.25 mm. (D) Western blot analyses of Ser70p-BCL2, BCL2, MCL1, Thr202/Tyr204p-ERK, and ERK protein expression levels in HL-60 cells after 24 hours of treatment with control vehicle, venetoclax plus azacitidine, or the triple combination. Only upon treatment with the triple combination were the levels of all 3 proteins p-BCL2, MCL1, and p-ERK significantly decreased. (E) Multidrug synergy graphs show the average ZIP score for double and triple combinations between FTY720, venetoclax, and azacitidine in 2 AML primary samples. Analyses were performed with the SynergyFinder Plus webtool. Synergy scores >10 (gray boxes) were considered synergistic. (F) Western blot analyses of Ser70p-BCL2, BCL2, MCL1, Thr202/Tyr204p-ERK, and ERK protein expression levels in AML-2 cells after 36 hours of treatment with venetoclax plus azacitidine or the triple combination. Only upon triple treatment was the expression levels of MCL1, Ser70p-BCL2, and Thr202/Tyr204p-ERK significantly decreased. (G) NSG mice were injected intravenously with AML-2 primary cells. Vehicle or double or triple-combination treatments were administrated by oral gavage starting 5 days postinjection. (H) Survival curve of an aggressive AML PDX model in NSG mice treated with vehicle, venetoclax plus azacitidine, or FTY720, venetoclax, and azacitidine combination (n = 7 mice per group). Triple-combination treatment induced a statistically significant increase in OS in treated mice compared with control animals (median OS, 57 vs 39 days; P = .007), or animals treated with venetoclax plus azacitidine (median OS, 47 vs 57 days; P = .01). Data were analyzed by the log-rank (Mantel-Cox) test. ZIP, zero interaction potency.
PP2A activation further improves the effectiveness of venetoclax plus azacitidine therapy in AML. (A) Multidrug synergy graphs show the average ZIP synergy score for double and triple combinations between FTY720, venetoclax, and azacitidine. Analyses were performed with the SynergyFinder Plus webtool. Synergy scores >10 (gray boxes) were considered synergistic. (B) Colonization potential of HL-60 cells in zebrafish xenograft models upon treatment with control, venetoclax plus azacitidine, or the triple therapy. Experiments were done in triplicate; number of embryos used is indicated as “n.” Data were analyzed by χ2 tests; ∗∗P < .01, ∗∗∗P < .001. (C) Representative images of HL-60 invading cells throughout the tail of zebrafish xenograft embryos at 72 hpi upon treatment with control, venetoclax plus azacitidine, or the triple therapy. AML cells were tracked as red-emitting fluorescent cells. Arrows show migrating HL-60 cells. Scale bar: 0.25 mm. (D) Western blot analyses of Ser70p-BCL2, BCL2, MCL1, Thr202/Tyr204p-ERK, and ERK protein expression levels in HL-60 cells after 24 hours of treatment with control vehicle, venetoclax plus azacitidine, or the triple combination. Only upon treatment with the triple combination were the levels of all 3 proteins p-BCL2, MCL1, and p-ERK significantly decreased. (E) Multidrug synergy graphs show the average ZIP score for double and triple combinations between FTY720, venetoclax, and azacitidine in 2 AML primary samples. Analyses were performed with the SynergyFinder Plus webtool. Synergy scores >10 (gray boxes) were considered synergistic. (F) Western blot analyses of Ser70p-BCL2, BCL2, MCL1, Thr202/Tyr204p-ERK, and ERK protein expression levels in AML-2 cells after 36 hours of treatment with venetoclax plus azacitidine or the triple combination. Only upon triple treatment was the expression levels of MCL1, Ser70p-BCL2, and Thr202/Tyr204p-ERK significantly decreased. (G) NSG mice were injected intravenously with AML-2 primary cells. Vehicle or double or triple-combination treatments were administrated by oral gavage starting 5 days postinjection. (H) Survival curve of an aggressive AML PDX model in NSG mice treated with vehicle, venetoclax plus azacitidine, or FTY720, venetoclax, and azacitidine combination (n = 7 mice per group). Triple-combination treatment induced a statistically significant increase in OS in treated mice compared with control animals (median OS, 57 vs 39 days; P = .007), or animals treated with venetoclax plus azacitidine (median OS, 47 vs 57 days; P = .01). Data were analyzed by the log-rank (Mantel-Cox) test. ZIP, zero interaction potency.
To confirm the mechanistic basis of the combined therapy, drug treatment and western blot analyses were performed on the AML-6, -7, and -11 samples. Consistent with the observations in cell lines, levels of p-BCL2, p-ERK, and MCL1 were significantly decreased upon treatment with FTY720 and venetoclax in therapy-responsive samples (AML-7 and AML-11), whereas no changes in phosphorylation or total protein levels were observed in the therapy-resistant sample (AML-6) (Figure 3F-G). Altogether, FTY720 with venetoclax induces potent and synergistic antileukemic effects ex vivo in primary AML cells from patients at high risk.
The PP2A-B56α complex is the master regulator of the observed treatment synergy
PP2A-B56α and PP2A-B55α complexes regulate most of the key signal transduction pathways involved in AML development and progression.16,17 To investigate their respective roles in the observed synergistic responses, we used CRISPR-Cas9 assays to generate HL-60 sublines lacking B56α or B55α subunits (Figure 4A-B; supplemental Figure 5A-B). Inactivation of B56α impeded changes in p-BCL2, p-ERK, and MCL1 protein levels following the combination therapy, thereby abolishing the synergistic effects (Figure 4C; supplemental Figure 5C-E). In contrast, B55α inactivation had no effect on either protein expression changes or cell viability (Figure 4C; supplemental Figure 5C-E).
These results pointed to a critical role for the PP2A-B56α complex in modulating the cytotoxic effects observed with combination treatment. Based on these results, we used DT-061, a PP2A activator that specifically stabilizes the PP2A-B56α holoenzyme, allowing it to dephosphorylate selective substrates.23 The combination of DT-061 plus venetoclax decreased p-BCL2, p-ERK, and MCL1 protein levels that induced synergistic antileukemic responses (supplemental Figure 6A-B). Moreover, HL-60 cells with inactivation of B56α showed no synergistic effects upon treatment with DT-061 and venetoclax (supplemental Figure 6C).
Accordingly, zebrafish models revealed that loss of B56α rendered HL-60 cells more resistant to the combination therapy in vivo, whereas inactivation of B55α did not change therapy responses (supplemental Figure 6D). Moreover, therapeutic experiments in immunodeficient NSG mice that underwent xenotransplantation with HL-60 wild-type B56α showed a significant reduction in tumor volume in the combined treatment group, which was not observed in mice carrying B56α-inactivated HL-60 cells (Figure 4D-E). Thus, FTY720 and venetoclax were synergistically active in vivo in NSG mice, which confirms our in vitro results and results from zebrafish models. Cells with loss of the B56α subunit had a slower in vivo growth rate and were resistant to either single-agent or combination treatment (Figure 4E). Furthermore, p-BCL2, p-ERK, and MCL1 levels increased in HL-60 cells with B56α inactivation upon the combination treatment (Figure 4F).
Next, we profiled the mRNA expression of PPP2R5A (gene coding for B56α) to investigate whether PPP2R5A mRNA levels could distinguish therapy responsive from nonresponsive leukemic cells. Cell lines resistant to the combination therapy had significantly lower PPP2R5A mRNA levels than sensitive cells. These results were confirmed in our series of patients with primary AML (Figure 4G), pointing to PPP2R5A as a potential biomarker of response to combined FTY720 and BH3 mimetics in AML.
Altogether, our results demonstrate that the PP2A-B56α complex drives the synergistic proapoptotic activity observed upon combination treatment in vitro and in vivo (Figure 4H), and that PPP2R5A could be a potential biomarker of FTY720 and BH3 mimetics treatment response in AML.
PP2A activation therapy enhances responses to clinically approved venetoclax-azacitidine combination in vitro, ex vivo, and in vivo
Venetoclax in combination with hypomethylating agents is the new standard treatment for older/unfit patients with AML.11 Based on our results, we hypothesized that a triple therapy consisting of FTY720-venetoclax-azacitidine might drive better efficacy than venetoclax-azacitidine. The triple combination had greater synergistic effects than venetoclax plus azacitidine in AML cell lines. (Figure 5A; supplemental Figure 7A). In this line, zebrafish xenograft models revealed that the triple therapy was significantly more effective than venetoclax plus azacitidine in reducing the colonization index of HL-60 cells (Figure 5B-C). Only the triple therapy led to a significant reduction in the levels of p-BCL2, p-ERK, and MCL1 proteins (Figure 5D). These results were confirmed with 2 additional PP2A activators, CM-1231 or DT-061, in combination with venetoclax-azacitidine (supplemental Figure 7B). Next, we tested this triple-combination therapy on primary AML cells, which was more effective than venetoclax plus azacitidine in AML-11 and AML-2 samples, and induced changes in p-BCL2, p-ERK, and MCL1 expression (Figure 5E-F).
We then examined the tolerability of this triple therapy. Ex vivo, the triple combination had minimal effects on normal human CD34+ hematopoietic stem/progenitor cells in CFU assays (supplemental Figure 8A-B). Furthermore, oral administration of FTY720-venetoclax-azacitidine for 4 weeks in NSG mice was not associated with weight loss or other indicators of sickness (supplemental Figure 8C-D). Accordingly, kidney and liver parameters and histological examination of stomach, kidney, and liver tissues showed no abnormalities in mice treated with the triple therapy compared with control mice (supplemental Figure 8E-F). Finally, CFU assays showed no differences in the growth of lineage-negative cells isolated from bone marrow of control and treated mice (supplemental Figure 8G-H). Overall, these results show that the triple combo is well tolerated in vivo.
Given that AML-2 cells carried adverse-risk mutations (supplemental Table 2), and that no synergy upon treatment with venetoclax and azacitidine was detected ex vivo (Figure 5E), these cells were selected to generate an aggressive mouse PDX model to analyze the efficiency of FTY720-venetoclax-azacitidine therapy in vivo. Cells were injected intravenously into NSG mice and treatment started 5 days after injection and continued for 8 weeks (Figure 5G). Mice treated with venetoclax plus azacitidine succumbed to disease at similar latency as vehicle-treated animals (median OS, 47 vs 39 days; P = .55). Conversely, mice treated with the triple combination showed a significant increase in OS compared with animals treated with venetoclax plus azacitidine (median OS, 57 vs 47 days; P = .01), or vehicle-treated animals (median OS, 57 vs 39 days; P = .007) (Figure 5H). These results reproduced ex vivo responses of primary AML-2 cells to combined venetoclax with azacitidine and our triple-combination therapy (Figure 5E,H). In summary, PP2A reactivation sensitized AML cells to venetoclax-mediated cytotoxicity and increased the efficacy of venetoclax plus azacitidine in vitro, ex vivo, and in vivo.
PP2A activation enhances the activity of BH3 mimetics in other hematological tumors
Next, we postulated that combination of FTY720 with BH3 mimetics might exhibit synergistic effects in other hematological malignancies in which PP2A is frequently inactivated.18 T-cell acute lymphoblastic leukemia cells were dependent on BCL-XL37 and synergistically responded to FTY720 plus A-1331852 (supplemental Figure 9A-D). Diffuse large B-cell lymphoma cell lines were dependent on BCL2 for survival38 and FTY720 plus venetoclax elicited synergistic responses (supplemental Figure 9A-B,E-F). Therefore, FTY720 and proapoptotic agents induce synergistic responses in these hematologic tumors when used with the appropriate BH3 mimetic drug.
Discussion
The VIALE-A trial reported that the venetoclax-azacitidine group had the longest OS in clinical trials for frontline older/unfit patients with AML.13 However, the long-term medical benefit is modest, and relapse appear unavoidable.5,14,15,39 Current data support the urgent need for testing novel venetoclax combinations. Here, we show that PP2A activators sensitized AML cells to venetoclax-mediated cytotoxicity, demonstrating that the PP2A-B56α holoenzyme is critical for the therapeutic synergy. PP2A-activating drugs increased the efficacy of venetoclax-azacitidine in vitro, in primary cells, and significantly prolonged mice survival in an AML PDX model.
Our results show that the antileukemic properties of combined FTY720 and venetoclax are associated with BCL2 dual inhibition, and decreased stability of MCL1, because of ERK inhibition by restoration of PP2A activity. Therefore, our novel drug combination antagonizes one of the most remarkable mechanisms of poor response to venetoclax-combined treatments, that is, high MCL1 expression levels.34 Preclinical studies show potent synergistic responses upon combination of venetoclax and MCL1 inhibitors;40 however, MCL1-targeting drugs have substantial toxicity to hematopoietic stem cells,41 hepatocytes,42 and cardiomyocytes.43 Our data and recent publications44,45 support that indirect induction of MCL1 downregulation is a more viable and well-tolerated therapeutic strategy.
Moreover, FTY720 plus venetoclax treatment efficiently inhibits the proliferation of AML cell lines and primary samples with high-risk mutations that are associated with poor response to venetoclax therapies.14 In fact, 78% of the primary samples that showed synergy to the combination had genetic alterations associated with adverse risk. Hence, our drug combination would be eligible for a broad group of AML cases, including patients classified in the adverse-risk group, and might prolong treatment response by overcoming the mechanisms of resistance to venetoclax combinations.
By using different PP2A activators and CRISPR-Cas9 genetic approaches, we highlight the function of PP2A-B56α in AML. We demonstrate that PP2A-B56α heterotrimers drive the combined therapy effects in AML in vitro and in vivo. The B56 family is linked to ERK dephosphorylation in cancer,33 and previous studies suggest that B56α regulates BCL2 in AML.31 Here, we prove that the PP2A-B56α heterocomplex dephosphorylates and inactivates both BCL2 and ERK in AML cell lines and primary cells. Of importance, high expression of PPP2R5A, the gene coding B56α, could be a potential biomarker to identify patients who would respond to this novel combination.
Venetoclax with azacitidine has been approved by both the US Food and Drug Administration and the European Medicines Agency for older/unfit patients with AML11 and represents the backbone of trials with novel compounds. We demonstrate that the antileukemic effect of combined venetoclax-azacitidine can be synergistically enhanced by PP2A activation in vitro, in primary AML cells, and in an aggressive AML PDX model. Of note, only the triple therapy led to a significant reduction in p-BCL2, p-ERK, and MCL1.
Throughout this study, we used 3 structurally different PP2A activators. To ensure that the observed synergistic effects are specifically because of PP2A reactivation,46 we include CM-1231, our novel nonphosphorylable analog of FTY720.20 Although the doses of FTY720 required to have cytotoxic effects are reduced when combined with venetoclax, CM-1231 has no cardiac toxicity.20 Therefore, combination of CM-1231 and venetoclax could be another clinically translatable option for patients with AML. FTY720 and CM-1231 reactivate PP2A by binding to SET, an endogenous PP2A inhibitor.20-22 However, to develop more personalized therapeutic strategies, the challenge is to find which specific PP2A complexes are affected in each disease. The identification of the key role of PP2A-B56α in the synergy led us to include DT-061, a small molecule that specifically stabilizes B56α-containing heterotrimers.23 Our results confirm the efficacy of DT-061 in AML, supporting the potential near-term clinical translation of PP2A modulators in the coming years.23 In fact, our proposal is not limited to AML; our data reveal that combination of PP2A activation with tailored BH3 mimetics could represent an attractive combinatorial strategy in other hematological malignancies.
In conclusion, our results demonstrate that PP2A reactivation enhances the efficacy of venetoclax and venetoclax-based combination therapies, and these encouraging results provide the rationale for the clinical development of new treatment strategies for AML based on the combination of PP2A-activating drugs with BH3 mimetics.
Acknowledgments
The authors thank Marta Larráyoz, Maddalen Jiménez, and Ismael Aizpún for useful discussion.
This work was supported by Instituto de Salud Carlos III – Acción Estratégica en Salud (PI17/02272, M.D.O.; PI20/01558, C.V. and M.D.O.; PI19/00818, J.A.M.-C.), CIBERONC (CB16/12/00489, E.A., J.A.M.-C., and M.D.O) and CIBERER (CB19/07/00031, M.L.C.) (cofinanced with FEDER funds), Department of Industry of the Government of Navarra (0011-1365-2016-000294, M.D.O.), and National Institutes of Health/National Cancer Institute grants (R01 CA-181654 and R01 CA-240993, G.N.). I.P. has received funding from “la Caixa” Banking Foundation and Asociación de Amigos (University of Navarra). S.R.-M. is supported by a grant from the Fundación para la Investigación Médica Aplicada (Programa de ayudas predoctorales de investigación biomédica AC FIMA). C.V. is supported by a grant from the Spanish Association Against Cancer (Fundación Científica AECC, INVES18061ODER). Some images used for figures were obtained under permission from the Somersault Library of Science and Medical Illustrations and Biorender.com.
Authorship
Contribution: I.P., M.D.O., and C.V. conceptualized the study; I.P., S.R.-M., E.M.-B., C.C.F., M.L.C., G.N., M.D.O., and C.V. designed the methodology; I.P., S.R.-M., E.M.-B., C.C.F., E.A., N.M., M.J.-M., and C.V. performed experiments; I.P., S.R.-M., E.M.-B., C.C.F., M.J.-M., and C.V. performed formal analyses; C.A.-P., A.T.-L., V.F., J.A.M.-C., and M.C.M. provided resources; M.L.C., G.N., M.D.O., and C.V. supervised the study; I.P., S.R.-M., V.F., and C.V. were responsible for visualization of data; I.P., M.D.O., and C.V wrote the original draft; and I.P., S.R.-M., V.F., J.A.M.-C., G.N., M.D.O., and C.V. wrote, reviewed, and edited the manuscript.
Conflict-of-interest disclosure: G.N. is chief scientific officer at RAPPTA Therapeutics, is a SAB member at Hera BioLabs, reports receiving commercial research support from RAPPTA Therapeutics, and has ownership interest (including patents) in RAPPTA Therapeutics, an asset development company developing small-molecule modulators of PP2A. The remaining authors declare no competing financial interests.
Correspondence: Carmen Vicente, Centro de Investigación Médica Aplicada, University of Navarra, Pio XII, 55, 31008 Pamplona, Spain; e-mail: cvicente@unav.es; and Maria D. Odero, Centro de Investigación Médica Aplicada, University of Navarra, Pio XII, 55, 31008 Pamplona, Spain; e-mail: modero@unav.es.
References
Author notes
All data associated with this study are present in the manuscript or the supplemental data file. Reagents and materials will be available upon reasonable request from the corresponding authors, Carmen Vicente (cvicente@unav.es) and Maria D. Odero (modero@unav.es).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
M.D.O. and C.V. are joint senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal