In this issue of Blood, Rao et al describe efficacy of the oral phosphoinositide 3-kinase delta (PI3Kδ) inhibitor leniolisib in patients with activated phosphoinositide 3-kinase delta syndrome (APDS).1
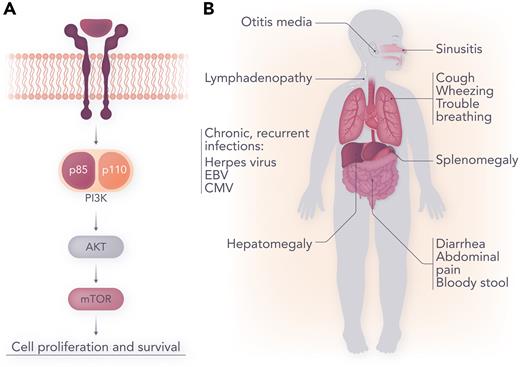
PI3Kδ is heterodimeric enzyme with critical subunits, p110δ and p85α. APDS is a primary immunodeficiency caused by a pathogenic variant in the PIK3CD gene, encoding the catalytic p110δ subunit (APSD1), or the PIK3R1 gene, encoding the regulatory p85α subunit (APSD2).2 Variants affecting p110δ lead to downstream hyperactivation of the AKT/mTOR/pS6K signaling pathway, whereas variants affecting p85α cause loss of p85α-mediated inhibition of p110δ activity, and therefore, constitutive activity of PI3Kδ (see figure panel A). Hyperactivation of the mTOR pathway—critical for cell growth, proliferation, differentiation, and survival—has many immunologic sequalae, including skewed differentiation of CD8+ T cells to short-lived effector cells, and impaired memory B- and T-cell development.3 Immune dysregulation is associated with hypogammaglobulinemia (often with conserved or increased immunoglobulin M levels), lymphopenia, and CD4+ T-cell reduction.
(A) Phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling. (B) Clinical manifestations of APDS. Professional illustration by Somersault18:24.
(A) Phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling. (B) Clinical manifestations of APDS. Professional illustration by Somersault18:24.
APDS was first described in 2013,3,4 and over 285 patients have been identified to date.5 Clinical features include recurrent sinopulmonary infections (pneumonia, sinusitis, otitis media), bronchiectasis, and viral infections [Epstein-Barr virus (EBV), cytomegalovirus, and other herpesviruses]. Benign lymphoproliferation, including bronchial and intestinal lymphoid hyperplasia, lymphadenopathy, splenomegaly, and hepatomegaly, is also common. The most frequent autoimmune manifestation is cytopenias, including Immune thrombocytopenia (ITP) and autoimmune hemolytic anemia. There is also increased risk of lymphoma, particularly among patients with a history of EBV infection. Finally, in a subset of patients (∼20%-30%) mild neurodevelopmental delay has been reported.6,7 (see figure panel B).
Management of APDS has traditionally included immunomodulatory agents (corticosteroids, rituximab, sirolimus), as well as prophylactic antimicrobials, immunoglobulin replacement, and consideration of hematopoietic stem cell transplant in severe cases. Leniolisib, an oral small molecule inhibitor which selectively blocks PI3Kδ, offers a compelling precision medicine approach.
This phase 3 study of leniolisib enrolled 31 patients from the United States, Europe and Russia, aged 12 to 54 years with known pathogenic variants in PIK3CD or PIK3R1, clinical findings of APDS, and 1 or more measurable lymph nodes. Patients were randomized 2:1 to receive 70 mg leniolisib or placebo twice daily. Individuals on immunosuppressive agents underwent a washout period before study entry. For the primary endpoint, patients were followed for 85 days to see if there was a negative change from baseline in the log10 transformed sum of product diameters of index measured lymph nodes. The second primary endpoint was a positive change from baseline after 85 days in the percentage of naïve B cells out of the total B cells assessed by flow cytometry. Several exploratory clinical, biomarker, and patient/physician reported outcomes were also examined as secondary endpoints. All patients were included in the safety analysis and 26 patients were included in the lymphadenopathy assessment (2 patients in each arm excluded because of protocol deviations; 1 patient was excluded because of full resolution of index node). In addition, 14 patients were excluded from the pharmacodynamic naïve B-cell endpoint owing to high naïve B cells at baseline, no follow-up assessments, or absent baseline measure of total B cells.
For the primary endpoint of lymphadenopathy reduction, adjusted mean change between leniolisib (n = 18) and placebo (n = 8) was −0.25 (95% confidence interval, −0.38 to −0.12; P = .0006). There was also suggestion of decreased spleen size in the leniolisib arm as compared with placebo. For the endpoint of improvement in immunodeficiency, proxied by increased percentage of naïve B cells, the difference in adjusted mean change between leniolisib and placebo from baseline to day 85 was 37.30 (95% confidence interval, 24.06-50.54; P = .0002). There were no statistically significant changes in patient- and clinician-reported outcomes. However, the authors note that investigator narratives describe positive improvements, including increased tolerance for physical activity and decreased fatigue in 70.0% of patients receiving leniolisib vs 44.4% receiving placebo. Safety data was reassuring. Most adverse events (AEs) were grade 1, and study drug-related AE incidence was lower in the leniolisib arm (23.8%) than the placebo arm (30.3%). Five patients reported serious AEs, none of which were judged as related to study medication. No deaths were reported.
The authors conclude that leniolisib given orally at 70 mg twice daily over 12 weeks reduced lymphadenopathy and increased naïve B-cell percentages significantly more than placebo. Furthermore, the drug was well tolerated.
Performing a phase 3 randomized clinical trial with a precision approach in patients with orphan diseases is extremely important. The authors should be commended for this effort as it sets a paradigm for other rare diseases that have similar clinical presentation but different disease biology. Although APDS is rare, a large number of genetic immune dysregulation disorders presenting with lymphoproliferation, autoimmunity, and risk of secondary malignancies have been identified over the past 20 years. These include ALPS (autoimmune lymphoproliferative syndrome; FAS pathway mutations), CHAI (CTLA4 haploinsufficiency with autoimmune infiltration; CTLA4 haploinsufficiency), LATAIE (LRBA deficiency with autoantibodies, regulatory T-cell defects, autoimmune infiltration, and enteropathy; LRBA mutations), and RALD (Ras-associated autoimmune leukoproliferative disorder; MAPK pathway mutations), as well as many others, and new disorders are described on frequent basis. Although there is substantial clinical overlap, the biologic drivers of lymphoproliferation and autoimmunity are distinct and targeted therapies are available for many of these conditions. Unfortunately, in most of these related disorders, data demonstrating efficacy of targeted therapies are anecdotal reports or nonrandomized trials.8
Rao et al demonstrated safety of leniolisib for treatment of APDS. Although it is laudable that the trial was successfully conducted with patients enrolled around the world with a rare disease, the primary endpoints—reduced lymphadenopathy and improved naïve B-cell count—may not clearly represent clinically meaningful results. It may be that these endpoints are indeed clinically impactful, but a 12-week follow-up period is not long enough to measure impact in a chronic disease with infectious and autoimmune complications. Importantly, patients enrolled on this trial have the option of continuing therapy through an open label extension (OLE) trial (NCT02859727) which will assess the safety and efficacy of long-term treatment. Results of the OLE are eagerly awaited, as leniosilib has potential to be a paradigm shifting targeted medicine for APDS.
Conflict-of-interest disclosure: D.T.T. serves on advisory boards for Sobi, Janssen, and BEAM Therapeutics; receives research funding from BEAM Therapeutics and NeoImmune Tech; and has patents pending on CD38 CAR-T cells for hematologic malignancies and biomarkers for cytokine release syndrome. H.N. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal