TO THE EDITOR:
The median age at immune thrombocytopenia (ITP) diagnosis in adult patients is ≈60 years,1,2 and ITP is not protective against thrombosis.3 Therefore, a significant proportion of adult patients with ITP are exposed to antiplatelet drugs,4 which is particularly challenging in clinical practice in regard to the risk of bleeding. In 2019, international guidelines for the management of ITP stated that a dedicated treatment is rarely indicated in case of platelet count >20 × 109/L.5 The threshold associated with significant bleeding in patients with ITP treated with antiplatelet agents is unknown.6 The international consensus recommended a platelet count target ≥30 × 109/L to 50 × 109/L in patients with ITP treated with a single antiplatelet agent, and a platelet count target ≥50 × 109/L to 70 × 109/L in patients with ITP treated by dual-antiplatelet treatment or single-antiplatelet agent + anticoagulant.5 However, these recommendations were based on expert opinion only. The 2019 American Society of Hematology guidelines for ITP management did not recommend a particular target in these patients but identified the delineation of bleeding risk of patients with ITP treated by antiplatelet agents as a priority unmet need in epidemiologic research in ITP.7 The aim of this study was to determine the frequency of bleeding by platelet counts in patients with ITP treated by antiplatelet drugs.
The source of data was the CARMEN (Cytopénies Auto-immunes : Registre Midi-pyrénéEN)-France registry, a prospective, real-world registry of adult patients with an incident diagnosis of ITP in France (NCT02877706).8 Inclusion criteria were adult patients (aged ≥18 years) newly diagnosed with ITP, according to the international definition (platelet count <100 × 109/L and exclusion of other causes of thrombocytopenia).9 Data quality assessment is made biyearly for a core set of variables in the registry, including those used in the present study, to check for missing or aberrant values. We designed a cross-sectional study to correlate the platelet count to bleeding manifestations at ITP diagnosis, before any ITP treatment to avoid any confounding by indication. The study population consisted of the patients included in the registry up to November 2021 and treated with antiplatelet agents at the time of ITP diagnosis, without concomitant exposure to anticoagulant. We measured the percentages of patients with overall bleeding, cutaneous bleeding, nonserious mucosal bleeding, and serious bleeding (defined by intracranial bleeding, gastrointestinal bleeding with anemia, or gross hematuria with anemia) by categories of platelet count (by 109/L up to 50 × 109/L, then ≥50 × 109/L). The analyses were conducted by 3 subgroups of antiplatelet agents because of differences in their pharmacodynamics: aspirin alone, clopidogrel alone, and dual-antiplatelet agents.
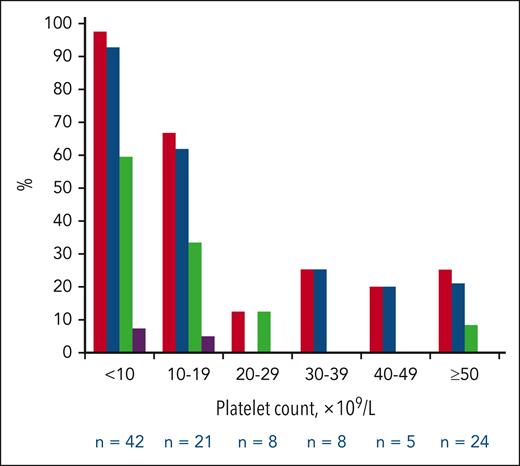
Of 1171 adult patients with an incident diagnosis of ITP included in the registry during the study period, 143 (12.2%) were exposed to an antiplatelet drug at the time of ITP diagnosis without any concomitant exposure to anticoagulant: 108 to aspirin alone, 20 to clopidogrel alone, and 15 to an association of antiplatelet agents (aspirin + clopidogrel, n = 13; and aspirin + ticagrelor, n = 2). Patients’ characteristics are described in supplemental Tables 1 to 3 (available on the Blood website). In the aspirin alone group, the median age was 75.5 years, 73 (67.6%) patients were men, the median platelet count at ITP diagnosis was 15 × 109/L, and 66 (61.1%) had bleeding: 60 (55.6%) had cutaneous bleeding, 35 (32.4%) had nonserious mucosal bleeding, and 4 (3.7%) had serious bleeding (3 gastrointestinal bleeding with anemia and 1 gross hematuria with anemia; described in supplemental Table 4). The pattern of bleeding by platelet count categories is shown in Figure 1. The frequency of any bleeding was 97.6% (41/42) in the <10 × 109/L platelet count category, 66.7% (14/21) in the 10 × 109/L to 19 × 109/L category, and 22.2% (10/45) in the ≥20 × 109/L category (similar pattern by all categories ≥20 × 109/L). The increased frequency of bleeding in patients with a platelet count <20 × 109/L and <10 × 109/L was also observed for cutaneous bleeding, nonserious mucosal bleeding, and serious bleeding. The 4 serious bleeding events occurred with a platelet count ≤10 × 109/L (supplemental Table 4). The receiver operating characteristic curve assessing the performance of platelet count as a continuous variable to discriminate the presence of any bleeding had an area under the curve of 88.3% (95% confidence interval, 81.9%-94.6%; supplemental Figure 1). The threshold of platelet count <20 × 109/L had a sensitivity of 83.3% and a specificity of 81.0%.
Frequencies of any bleeding, cutaneous bleeding, nonserious mucosal bleeding, and serious bleeding by platelet count categories in the group of patients treated with aspirin alone (n = 108). Red bars, any bleeding; blue bars, cutaneous bleeding; green bars, nonserious mucosal bleeding; and purple bars, serious bleeding.
Frequencies of any bleeding, cutaneous bleeding, nonserious mucosal bleeding, and serious bleeding by platelet count categories in the group of patients treated with aspirin alone (n = 108). Red bars, any bleeding; blue bars, cutaneous bleeding; green bars, nonserious mucosal bleeding; and purple bars, serious bleeding.
In the clopidogrel group and in the dual-antiplatelet drug group, bleeding was observed in 14 (70.0%) and 10 (66.7%) patients, respectively. No correlation with the platelet count was shown because of the low number of patients (supplemental Figures 2 and 3). One serious bleeding event occurred in each group (2 intracranial bleeding events), in patients with platelet counts <10 × 109/L (supplemental Tables 5 and 6).
In this cross-sectional study, the pattern of bleeding by platelet count categories in patients treated by aspirin was similar to the pattern observed in other patients with ITP.10,11 A >50% frequency of bleeding was also observed in this registry in case of platelet count <20 × 109/L in patients not exposed to antiplatelet or anticoagulant drugs (supplemental Figure 4). In a previous study in the CARMEN-France registry, the exposure to aspirin was not associated with bleeding at ITP diagnosis in multivariable analysis.10 A potential explanation might be that aspirin is a nonreversible ligand of cyclooxygenase 1.12 In ITP, platelets with bound aspirin may be rapidly cleared by the immune system and replaced by new platelets produced by the bone marrow. On the other hand, these results raise the question of sufficient cardiovascular protection by aspirin in patients with ITP with a low risk of bleeding and a high cardiovascular risk, as demonstrated in other diseases with a high platelet turnover.13
The main limitation of this study is that the low number of patients in the clopidogrel alone group and in the dual-antiplatelet drug group prevented from any determination of platelet count threshold associated with significant bleeding in these groups. Clopidogrel is a nonreversible ligand of P2Y12, and ticagrelor is a reversible ligand of P2Y12, with longer half-lives than aspirin.12 Therefore, the frequencies of bleeding may be higher than those observed with aspirin alone. Two intracranial bleeding events were observed in these groups, in patients with a platelet count <10 × 109/L. In the future, the combination of registries may be a way to select more patients corresponding to these subgroups and answer this question.14 This may not be the same in patients exposed to anticoagulant alone, or to antiplatelet drugs + anticoagulant. Indeed, the exposure to anticoagulant has been associated with serious bleeding, particularly in older patients,10,15 and the threshold of platelet count to avoid bleeding in these patients is still to be determined.7
This study was designed as a cross-sectional one to avoid confounding by ITP treatment in the platelet count threshold determination. Therefore, other cohort studies with longitudinal analysis are now needed to assess whether maintaining a platelet count >20 × 109/L is safe in patients with ITP and treated with aspirin alone.
In conclusion, this study determined a threshold of platelet count <20 × 109/L significantly associated with bleeding in patients with ITP treated with aspirin alone, as in most patients with ITP not exposed to aspirin.
Acknowledgments
The authors thank all the patients and investigators. The authors also thank the sponsors of the CARMEN-France registry: Toulouse University Hospital, the French National Society of Internal Medicine, Amgen SAS, CSL Behring SAS, Grifols SAS, and Novartis SAS. The authors thank all research assistants who acquired the data: Cédric Castex (Reims), Astrid de Rendinger (Paris), Elodie Deruche (Amiens), Quentin Ducrocq (Lille), Hélène Duval (Caen), Fatima Farhi (Paris), Céline Feugere (Clermont-Ferrand), Nelly Francois (Nancy), Cindy Fraysse (Toulouse), Fanny Gallo (Nancy), Claudia Gillet (Toulouse), Amandine Hubert (Amiens), Aude Jouinot (Périgueux), Laetitia Languille (Paris), Carine Lopez (Bordeaux), Nathalie Marchand (Paris), Emilie Mathiotte (Lyon), Chloe McAvoy (Paris), Kewin Panel (Paris), Marie Pereira (Paris), Valérie Predan (Dijon), Onja Rarison (Marseille), Jessica Rousson (Lyon), Jamila Sahraoui (Montpellier), Nithurya Uthayakumar (Paris), and Joanne Velazquez (Toulouse, Saint Exupery Private Hospital). The authors thank the coordination team of the registry: Johanne Germain, Laurie Chabbert, Marianne Navarra, Charline Daguzan, Christophe Morin, Milena Preti, Manuela Rueter, Marie-Aline Sarda, and Agnès Sommet.
Authorship
Contribution: N.O., M.-L.-P.-J., M.L.-M., and G.M. designed the study; N.O., M.-L.-P.-J., and G.M. conducted the statistical analyses and wrote the manuscript; all other authors contributed to the inclusion of patients, data collection, variable checking, and interpretation of the results; and all authors critically reviewed the manuscript and gave final approval.
Conflict-of-interest disclosure: This study was academic. The registry has been or is funded by Amgen SAS, CSL Behring SAS, Grifols SAS, and Novartis SAS. These companies had no access to data and no role in analysis, interpretation of results, and manuscript writing. G.M. received meeting attendance grants from Amgen, Grifols, and Novartis; and is coordinator of research studies granted by Amgen, CSL Behring, Novartis, and Grifols. He participated in educational sessions funded by Amgen, Grifols, and Novartis; and on boards for Amgen, Argenx, Grifols, Novartis, Sanofi, and Sobi. T.C. received honoraria and/or research or educational support from AbbVie, AstraZeneca, Bristol Myers Squibb (Celgene), Novartis, and Takeda. S.C. is investigator of research studies granted by Bioverativ, Novartis, Protalex, and Rigel; and participated on boards for Novartis and Sobi. M.E. received meeting attendance grants from Novartis, Octapharma, and Sobi; and participated in educational sessions for Amgen, Grifols, and Novartis and on boards for Grifols and Novartis. M. Michel participated in educational sessions and boards for Amgen, Argenx, Novartis, Sobi, and UCB. B.G. participated in educational sessions and boards for Amgen, Grifols, Novartis, Roche, and Sobi. The remaining authors declare no competing financial interests.
A complete list of the members of the CARMEN-France investigators group appears in the supplemental Material.
Correspondence: Guillaume Moulis, Service de Médecine Interne, Centre Hospitalier Universitaire de Toulouse, France, Place du Docteur Baylac, TSA 40031, 31059 Toulouse Cedex 9, France; e-mail: moulis.g@chu-toulouse.fr.
References
Author notes
Data may be obtained from a third party and are not publicly available. The data management and statistical analysis code is available on reasonable request to the corresponding author.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal