Key Points
Geriatric assessments can aid in identifying patients with less physical resilience who are at increased risk of grade ≥3 adverse events.
Fixed-duration Ven-O improves HRQoL in patients with CLL with and without geriatric impairments.
Abstract
Chronic lymphocytic leukemia (CLL)–related symptoms and morbidity related to the advanced age at diagnosis impairs the well-being of older adult patients. Therefore, it is essential to tailor treatment according to geriatric characteristics and aim for an improvement in health-related quality of life (HRQoL) as a primary treatment goal. In the HOVON139/GiVe trial, 12 cycles of fixed-duration venetoclax plus obinutuzumab (Ven-O) were shown to be effective and tolerable in FCR (fludarabine, cyclophosphamide, rituximab)-unfit patients with CLL (n = 67). However, prolonged venetoclax exposure as consolidation treatment led to increased toxicity with limited effect on minimal residual disease. To assess the impact of geriatric assessment on treatment outcomes and the patients’ HRQoL, patient-reported outcomes (PROs), including function, depression, cognition, nutrition, physical performance, muscle parameters, comorbidities, and the European Organization for Research and Treatment of Cancer C30 and CLL17 questionnaires were assessed. At baseline, geriatric impairments were present in >90% of patients and ≥2 impairments present in 60% of patients predicted grade ≥3 nonhematological toxicity. During treatment, the number of geriatric impairments diminished significantly and clinically relevant improvements in HRQoL subscales were reached for global health status, physical functioning, role functioning, emotional functioning, fatigue, dyspnea, physical condition or fatigue, and worries or fears related to health and functioning. These improvements were comparable for patients receiving venetoclax consolidation and patients in whom treatment could mostly be discontinued. Collectively, frontline fixed-duration Ven-O improves overall PROs in older, unfit patients with CLL with and without geriatric impairments. This study was registered at EudraCT as 2015-004985-27 and the Netherlands Trial Register as NTR6043.
Introduction
Age-standardized incidence rate of patients with chronic lymphocytic leukemia (CLL) ranges from 3.8 to 5.0 per 100 000 person-years.1-4 The number of cases rises with age, reaching 31.0 and 34.5 per 100 000 person-years for patients aged from 70 to 80 years and ≥80 years, respectively.1 Older patients are highly heterogeneous with regard to their process of aging including variability in comorbidities, cognitive and locomotive function, and their capability to perform activities in daily living.5-7 Geriatric assessments (GAs) is a multidisciplinary diagnostic evaluation that identifies medical, psychosocial, and functional impairments in order to guide planned therapy and arrange for targeted interventions to support these vulnerabilities in older patients receiving cancer treatment. Although recommended by the International Society of Geriatric Oncology, GA is not routinely implemented in the daily practice.8
Over the past decade, numerous targeted approaches were introduced particularly for older patients with CLL with coexisting comorbidities who are unable to tolerate intensive chemoimmunotherapy with FCR (fludarabine, cyclophosphamide, and rituximab).9-23 Less toxic strategies, such as chlorambucil-obinutuzumab or bendamustine-rituximab, were previously developed to be more tolerable but did not achieve similar efficacy.24,25 The CLL14 trial, including older patients with CLL with coexisting comorbidities, demonstrated a considerably higher undetectable minimal residual disease (MRD) rate and prolonged progression-free survival (PFS) with targeted agents venetoclax-obinutuzumab (Ven-O) than that with conventional chemoimmunotherapy with chlorambucil-obinutuzumab.10 Recently, the HOVON139/GiVe trial has demonstrated that prolongation of venetoclax exposure as consolidation treatment increased the duration of well-known side effects and did not prevent the loss of MRD response and subsequent risk of disease relapse.26
Treatment advanced with chemoimmunotherapy and targeted therapies contributed to the improved life expectancy of older patients with CLL.27 However, particularly among older patients with CLL, a central goal of therapy should be to enable patients to live their remaining life-years, while preserving good function and quality of life (QoL). At present, studies on the combined analysis of GA and health-related QoL (HRQoL) in older patients with CLL treated with novel approaches are lacking. Consequently, to evaluate the impact of Ven-O on the patients’ QoL and further understand the impact of GA on treatment outcomes and HRQoL, patient-reported outcomes (PROs) were collected in the HOVON139/GiVe trial.26 In this study, we report on all the collected domains of the GA and the HRQoL subscales.
Methods
Patient population and study design
The HOVON 139/GiVe is a multicenter, randomized, parallel group phase 2 trial. Details on the study design, eligibility criteria, and main outcomes of the study have been outlined previously.26 In short, patients with previously untreated symptomatic CLL requiring treatment according to the International workshop on Chronic Lymphocytic leukemia criteria,28 with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and who were considered to be unfit for first-line fludarabine-based treatment according to their treating physician, were enrolled. The study treatment consisted of 3 phases: preinduction for debulking with 2 cycles of IV obinutuzumab monotherapy (100 mg on day 1, 900 mg on day 2, and 1000 mg on days 8 and 15, and subsequently 1000 mg on day 1 of cycle 2), induction with 12 cycles of fixed-duration Ven-O (6 cycles of IV obinutuzumab 1000 mg on day 1 and 12 cycles of oral venetoclax starting with a weekly ramp-up schedule of 20 mg, 50 mg, 100 mg, 200 mg, and subsequently 400 mg until the end of cycle 12), and consolidation with either fixed 12 cycles of oral venetoclax (400 mg) or MRD-guided venetoclax (until undetectable MRD was reached or for a maximum of 12 cycles).
All patients were included by their treating physician, and all patients provided written informed consent. The study was approved by the Medical Ethical Committee of the Academic Medical Center of Amsterdam and was conducted in accordance with the Declaration of Helsinki.
PRO measures
The GA consisted of the following tools: the Charlson Comorbidity Index,29 the Katz Activity of Daily Living (ADL),30 the Lawton Instrumental Activity of Daily Living (IADL),31 the Geriatric Depression Scale 15,32 the Mini Nutritional Assessment Short Form,33 and the mini mental state examination.34 The European Working Group on Sarcopenia in Older People 2010 algorithm and the 2018 algorithm were used to evaluate sarcopenia.35,36 Grip strength was used as a proxy for muscle strength, gait speed for muscle performance, and skeletal muscle index and muscle radiation attenuation for muscle mass and muscle quality, respectively. HRQoL was assessed using 2 European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaires: the cancer-specific C30 and the CLL-specific CLL17.37,38 Further details on the used PROs are provided in the supplemental Methods, available on the Blood website. All assessments were performed at baseline (T0), after 12 induction cycles (T1), and 15 months after randomization (T2) whereas an addition HRQoL assessment was performed at 12 months after completion of protocol treatment (T3).
Statistical analysis
Descriptive analysis was performed for the patients at baseline and their GA characteristics. Correlations between individual geriatric domains was assessed using the Spearman correlation coefficient ρ. The association of the number of geriatric impairments at baseline with adverse events (AEs), PFS, and overall survival (OS) was evaluated using cumulative incidence and Kaplan Meier analysis. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and grade ≥3 hematological and nonhematological AEs were presented. The longitudinal evolution of the number of geriatric impairments was assessed using linear mixed-effect models.
For the HRQoL analysis, mean scores and the 95% confidence interval of each subscale were calculated according to the EORTC manual.39 Trends over time for the overall cohort and according to the treatment consolidation arm were assessed using linear mixed-effect models. Distribution-based minimal important differences (MIDs) were calculated to determine whether an improvement or deterioration in HRQoL was considered to be clinically relevant.40,41 MID thresholds for clinical relevance between arms was defined as >5 points difference at a specific time point.42 For QLQ-C30 subscales, an additional anchor-based method was used to assess whether the effect size of the change in HRQoL was either trivial, small, medium, or large.43,44 Effect modification across the HRQoL subscales by the number of geriatric impairments (ie, 0-1 and ≥2 impairments) was assessed using linear mixed-effect models. Details regarding the linear mixed-effect models and the MIDs are provided in the supplemental Methods. A P value < .005 was considered statistically significant, because multiple subscales were tested.45 All statistical analyses were performed with STATA Statistical Software version 17.0 (StataCorp, College Station, TX) and R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria). This study is ongoing and is registered at EudraCT (2015-004985-27) and the Netherlands Trial Register (NTR6043).
Results
Patient characteristics
The HOVON139/GiVe trial enrolled 70 patients, of whom 3 were excluded because of ineligibility upon hindsight (supplemental Figure 1). Current data were analyzed using a cut-off date of 1 September 2022, with a median follow-up time for the overall cohort of 44 months (interquartile range 38-51). Sixty-seven patients received preinduction with obinutuzumab for debulking followed by fixed-duration induction treatment with Ven-O of whom 5 discontinued due to death (n = 1), withdrawal of consent (n = 1), or excessive toxicity (n = 3). Consequently, 62 patients were randomly assigned to receive 12 cycles venetoclax consolidation (n = 32) or MRD-guided venetoclax consolidation (n = 30). In the MRD-guided venetoclax consolidation arm, 1 (3%) of 30 patients was tested positive for MRD at randomization and received venetoclax consolidation cycles 1 to 3 until MRD-negativity was reached. All the remaining patients tested as MRD undetectable and did not receive any consolidation, although 1 patient with undetectable MRD incorrectly received venetoclax consolidation cycles 4 to 12 after MRD conversion.26 Therefore, 93% of the patients in this arm remained without any consolidation treatment during this phase of the protocol. Patient characteristics for the overall cohort and according to the consolidation arm are described in Table 1. Baseline characteristics were comparable for patients who received 12 cycles of venetoclax consolidation, MRD-guided venetoclax consolidation, and for those who were not randomly assigned.26
Baseline patient characteristics for the overall cohort and according to the consolidation arm
| Characteristics . | Overall . | Consolidation arm . | ||||
|---|---|---|---|---|---|---|
| 12 cycles venetoclax . | MRD-guided venetoclax . | |||||
| No. . | % . | No. . | % . | No. . | % . | |
| Total patients | 67 | 32 | 30 | |||
| Age | ||||||
| Median (IQR) | 71 (68-75) | 72 (69-75) | 71 (68-74) | |||
| 18-70 | 21 | 31 | 9 | 28 | 12 | 40 |
| >70 | 46 | 69 | 23 | 72 | 18 | 60 |
| Sex | ||||||
| Male | 47 | 70 | 24 | 75 | 20 | 67 |
| Female | 27 | 30 | 8 | 25 | 10 | 33 |
| ECOG performance status | ||||||
| 0 | 35 | 52 | 17 | 53 | 14 | 47 |
| 1 | 29 | 43 | 14 | 44 | 14 | 47 |
| 2 | 3 | 5 | 1 | 3 | 2 | 7 |
| Cumulative illness rating scale | ||||||
| Median (IQR) | 3 (1-5) | 3 (1-5) | 3 (1-5) | |||
| ≥7 | 13 | 20 | 6 | 19 | 6 | 20 |
| Binet stage | ||||||
| A | 9 | 13 | 6 | 19 | 3 | 10 |
| B | 26 | 39 | 14 | 44 | 11 | 37 |
| C | 32 | 48 | 12 | 38 | 16 | 53 |
| IGHV mutational status | ||||||
| Mutated | 26 | 39 | 13 | 41 | 11 | 37 |
| Unmutated | 33 | 49 | 17 | 53 | 14 | 47 |
| Unknown | 8 | 12 | 2 | 6 | 5 | 17 |
| TP53 aberrations | 9 | 13 | 5 | 16 | 4 | 13 |
| Genomic complexity∗ | ||||||
| None | 52 | 78 | 25 | 78 | 23 | 77 |
| Low | 10 | 15 | 5 | 16 | 5 | 17 |
| High | 5 | 7 | 2 | 6 | 2 | 7 |
| Hemoglobulin concentration, g/dL | ||||||
| Median (IQR) | 11 (10-13) | 12 (10-13) | 11 (10-13) | |||
| Platelet count, ×109cells per L | ||||||
| Median (IQR) | 134 (91-212) | 143 (109-210) | 111 (79-220) | |||
| White blood cell count, ×109cells per L | ||||||
| Median (IQR) | 110 (65-217) | 82 (55-175) | 79 (9-159) | |||
| Lymphocyte count, ×109cells per L | ||||||
| Median (IQR) | 94 (55-175) | 95 (62-160) | 82 (55-175) | |||
| β2 microglobin concentration, mg/dL | ||||||
| Median (IQR) | 4 (3-5) | 4 (4-5) | 4 (3-6) | |||
| Creatinine clearance, mL/min | ||||||
| Median (IQR) | 73 (60-83) | 71 (57-79) | 73 (65-87) | |||
| <70 | 29 | 43 | 15 | 47 | 12 | 40 |
| CLL-IPI risk group | ||||||
| Low risk | 2 | 3 | 0 | 0 | 2 | 7 |
| Intermediate risk | 10 | 15 | 8 | 25 | 2 | 7 |
| High risk | 39 | 58 | 18 | 56 | 18 | 60 |
| Very high risk | 9 | 13 | 5 | 16 | 4 | 13 |
| Unknown | 7 | 10 | 1 | 3 | 4 | 13 |
| Follow-up time, mo | ||||||
| Median (IQR) | 44 (38-51) | 46 (38-52) | 44 (39-50) | |||
| Characteristics . | Overall . | Consolidation arm . | ||||
|---|---|---|---|---|---|---|
| 12 cycles venetoclax . | MRD-guided venetoclax . | |||||
| No. . | % . | No. . | % . | No. . | % . | |
| Total patients | 67 | 32 | 30 | |||
| Age | ||||||
| Median (IQR) | 71 (68-75) | 72 (69-75) | 71 (68-74) | |||
| 18-70 | 21 | 31 | 9 | 28 | 12 | 40 |
| >70 | 46 | 69 | 23 | 72 | 18 | 60 |
| Sex | ||||||
| Male | 47 | 70 | 24 | 75 | 20 | 67 |
| Female | 27 | 30 | 8 | 25 | 10 | 33 |
| ECOG performance status | ||||||
| 0 | 35 | 52 | 17 | 53 | 14 | 47 |
| 1 | 29 | 43 | 14 | 44 | 14 | 47 |
| 2 | 3 | 5 | 1 | 3 | 2 | 7 |
| Cumulative illness rating scale | ||||||
| Median (IQR) | 3 (1-5) | 3 (1-5) | 3 (1-5) | |||
| ≥7 | 13 | 20 | 6 | 19 | 6 | 20 |
| Binet stage | ||||||
| A | 9 | 13 | 6 | 19 | 3 | 10 |
| B | 26 | 39 | 14 | 44 | 11 | 37 |
| C | 32 | 48 | 12 | 38 | 16 | 53 |
| IGHV mutational status | ||||||
| Mutated | 26 | 39 | 13 | 41 | 11 | 37 |
| Unmutated | 33 | 49 | 17 | 53 | 14 | 47 |
| Unknown | 8 | 12 | 2 | 6 | 5 | 17 |
| TP53 aberrations | 9 | 13 | 5 | 16 | 4 | 13 |
| Genomic complexity∗ | ||||||
| None | 52 | 78 | 25 | 78 | 23 | 77 |
| Low | 10 | 15 | 5 | 16 | 5 | 17 |
| High | 5 | 7 | 2 | 6 | 2 | 7 |
| Hemoglobulin concentration, g/dL | ||||||
| Median (IQR) | 11 (10-13) | 12 (10-13) | 11 (10-13) | |||
| Platelet count, ×109cells per L | ||||||
| Median (IQR) | 134 (91-212) | 143 (109-210) | 111 (79-220) | |||
| White blood cell count, ×109cells per L | ||||||
| Median (IQR) | 110 (65-217) | 82 (55-175) | 79 (9-159) | |||
| Lymphocyte count, ×109cells per L | ||||||
| Median (IQR) | 94 (55-175) | 95 (62-160) | 82 (55-175) | |||
| β2 microglobin concentration, mg/dL | ||||||
| Median (IQR) | 4 (3-5) | 4 (4-5) | 4 (3-6) | |||
| Creatinine clearance, mL/min | ||||||
| Median (IQR) | 73 (60-83) | 71 (57-79) | 73 (65-87) | |||
| <70 | 29 | 43 | 15 | 47 | 12 | 40 |
| CLL-IPI risk group | ||||||
| Low risk | 2 | 3 | 0 | 0 | 2 | 7 |
| Intermediate risk | 10 | 15 | 8 | 25 | 2 | 7 |
| High risk | 39 | 58 | 18 | 56 | 18 | 60 |
| Very high risk | 9 | 13 | 5 | 16 | 4 | 13 |
| Unknown | 7 | 10 | 1 | 3 | 4 | 13 |
| Follow-up time, mo | ||||||
| Median (IQR) | 44 (38-51) | 46 (38-52) | 44 (39-50) | |||
CLL-IPI, CLL International Prognostic Index; IGHV, immunoglobulin heavy chain variable.
Genomic complexity was defined as none in case of 0 or 2 copy number aberrations, low for 3 or 4 copy number aberrations, and high for ≥5 copy number aberrations.
Compliance
All patients (n = 67, 100%) who started treatment in the framework of the HOVON139/GiVe trial, provided informed consent for participating in the GA and HRQoL study. The compliance to GA for patients who were on protocol was 67 of 67 (100%) at baseline (T0), 60 of 66 (91%) after 12 induction cycles (T1), 26 of 32 (81%) for patients assigned to 12 cycles of venetoclax consolidation, and 24 of 30 (80%) for patients assigned to MRD-guided venetoclax consolidation at 15 months after randomization (T2) (supplemental Figure 1). At T1, the GA was incomplete in 3% and not performed in 6%. The corresponding percentages at T2 were 15% and 5%, respectively (supplemental Figure 2). Incompleteness of the GA was exclusively because of unavailability of functional assessment data. The compliance to HRQoL questionnaires was 67 of 67 (100%) at T0, 63 of 66 (95%) at T1, 31 of 32 (97%) for patients assigned to 12 cycles venetoclax consolidation, and 28 of 30 (93%) for patients assigned to MRD-guided venetoclax consolidation at T2. The corresponding compliances performed at 12 months after completion of protocol treatment (T3) were 23 of 28 (82%) and 23 of 30 (77%), respectively.
GA
Geriatric impairments at baseline
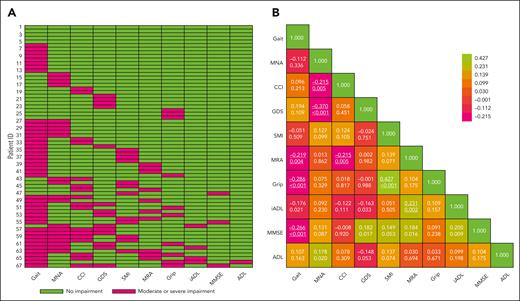
At baseline, the median number of geriatric impairments was 2 (interquartile range 1-3). Overall, ≥1 geriatric impairments were present in >90% of the patients including impairments in gait speed (64%), nutrition (31%), depression (21%), muscle mass (18%), grip strength (15%), muscle density (15%), cognition (4%), and the capability to perform instrumental activities and activities in daily living (4% and 1%, respectively; Figure 1A; supplemental Table 1). Sarcopenia, defined according to the European Working Group on Sarcopenia in Older People 2010 algorithm and the 2018 algorithm, was present in 6% and 1% of the patients, respectively. Seventeen patients (25%) had a moderate-to-severe comorbidity burden as defined by a Charlson Comorbidity Index score ≥2. The most prevalent comorbidities were chronic pulmonary disease (13%), moderate or severe renal disease (12%), and cerebrovascular disease (8%) (supplemental Table 2). Correlations were found between (1) gait speed and grip strength (P < .001), mini mental state examination (P < .001) or muscle radiation attenuation (P = .004), (2) mini nutritional assessment short form and depression (P < .001) or comorbidities (P = .005), (3) muscle density and comorbidities (P = .005) or IADL (P = .002), and (4) muscle mass and grip strength (P < .001). Overall, these correlations were graded as moderate-to-weak, according to the Spearman correlation coefficient ρ. All correlations among the individual geriatric domains are depicted in Figure 1B. Notably, the patients’ ECOG performance status weakly correlated with the number of geriatric impairments at baseline (ρ, 0.289; P < .001).
Baseline GA characteristics. (A) The prevalence of moderate or severe geriatric impairments across the individual patients. Patients were sorted according to the number of impairments (ie, from 0-5 impairments), and assessments were arranged based on the prevalence of impairments in the individual domains. Moderate or severe impairments were defined as a gait speed >0.80 m/s, MNA between 8 and 11 and <7, CCI ≥ 2, GDS > 5, SMI ≤ 32.0 cm2/m2 for females and ≤ 41.6 cm2/m2 for males, MRA ≤ 22.0 HU for females and ≤ 29.3 HU for males, grip strength <16 for females and <27 kg for males, IADL <5 for females and <8 for females, MMSE < 24, and ADL < 5. (B) Correlation between the individual domains of the GA. The numbers in the cells denote the Spearman rank correlation coefficient (Spearman correlation coefficient ρ) (top) and the corresponding P values (bottom). The color implies the direction and strength of the correlations. A positive ρ describes a positive correlation, whereas a negative ρ describes an inverse correlation. The effect size of the Spearman rank correlation coefficient can be verbally described using the following guide for the ρ: from 0.00 to 0.10, very weak; from 0.20 to 0.39, weak; from 0.40 to 0.59, moderate; from 0.60 to 0.79, strong; and from 0.80 to 1.00, very strong. Significant P values < .005 are underlined. ADL, Katz Activity of Daily Living; CCI, Charlson Comorbidity Index; GDS, Geriatric Depression Scale 15; MNA, Mini Nutritional Assessment; MMSE, Mini Mental State Examination; MRA, muscle radiation attenuation; SMI, skeletal muscle index.
Baseline GA characteristics. (A) The prevalence of moderate or severe geriatric impairments across the individual patients. Patients were sorted according to the number of impairments (ie, from 0-5 impairments), and assessments were arranged based on the prevalence of impairments in the individual domains. Moderate or severe impairments were defined as a gait speed >0.80 m/s, MNA between 8 and 11 and <7, CCI ≥ 2, GDS > 5, SMI ≤ 32.0 cm2/m2 for females and ≤ 41.6 cm2/m2 for males, MRA ≤ 22.0 HU for females and ≤ 29.3 HU for males, grip strength <16 for females and <27 kg for males, IADL <5 for females and <8 for females, MMSE < 24, and ADL < 5. (B) Correlation between the individual domains of the GA. The numbers in the cells denote the Spearman rank correlation coefficient (Spearman correlation coefficient ρ) (top) and the corresponding P values (bottom). The color implies the direction and strength of the correlations. A positive ρ describes a positive correlation, whereas a negative ρ describes an inverse correlation. The effect size of the Spearman rank correlation coefficient can be verbally described using the following guide for the ρ: from 0.00 to 0.10, very weak; from 0.20 to 0.39, weak; from 0.40 to 0.59, moderate; from 0.60 to 0.79, strong; and from 0.80 to 1.00, very strong. Significant P values < .005 are underlined. ADL, Katz Activity of Daily Living; CCI, Charlson Comorbidity Index; GDS, Geriatric Depression Scale 15; MNA, Mini Nutritional Assessment; MMSE, Mini Mental State Examination; MRA, muscle radiation attenuation; SMI, skeletal muscle index.
The association of geriatric impairments with treatment toxicity and outcome
The cumulative incidence of grade ≥3 AEs was higher for patients with ≥2 geriatric impairments than for those with 0 or 1 geriatric impairment (99% vs 62%; P < .001; supplemental Figure 3). The higher cumulative incidence was due to more nonhematological AEs (70% in patients with ≥2 impairments vs 34% for patients with 0-1 impairment; P < .001), whereas the incidence of hematological AEs was comparable (58% vs 54%; P = .487). An overview of the type of grade ≥3 AEs and occurrence of dose reductions are depicted in supplemental Table 3. The PFS rate (81% vs 82%; P = .819) and OS rate (96% vs 95%; P = .846) at 48 months were comparable for patients with 0 or 1 and ≥2 geriatric impairments, respectively (supplemental Figure 3).
Longitudinal changes in the GA
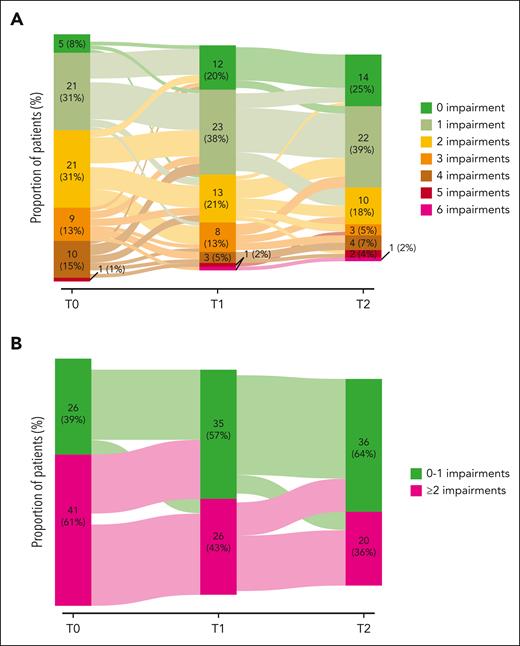
During the course of the treatment, the number of geriatric impairments per individual decreased significantly (Ptrend < .001; Figure 2A). More specifically, the number of patients with ≥2 geriatric impairments decreased from 61% at T0 to 43% at T1 and 36% at T2 (Figure 2B). The trends in the geriatric impairments per individual over the different domains and the prevalence of sarcopenia over time are depicted in supplemental Figures 4 and 5, respectively. The improvements in the GA were irrespective of the receipt of 12 cycles of venetoclax consolidation treatment compared with MRD-guided venetoclax consolidation (supplemental Table 4).
Sankey plots for the change in the number of geriatric impairments over time. (A) Absolute number of geriatric impairments over time. (B) Categorization of the number of geriatric impairments in 0-1 and ≥2 geriatric impairments at baseline. Of note, the cut-off was established based on the median number of geriatric impairments at baseline. The absolute number of geriatric impairments and the patients with ≥2 geriatric impairments significantly diminished over time (P < .001), as measured using a linear mixed-effect model, with the number of geriatric impairments and their 2-way interaction with time as a fixed effect and a random intercept for patients.
Sankey plots for the change in the number of geriatric impairments over time. (A) Absolute number of geriatric impairments over time. (B) Categorization of the number of geriatric impairments in 0-1 and ≥2 geriatric impairments at baseline. Of note, the cut-off was established based on the median number of geriatric impairments at baseline. The absolute number of geriatric impairments and the patients with ≥2 geriatric impairments significantly diminished over time (P < .001), as measured using a linear mixed-effect model, with the number of geriatric impairments and their 2-way interaction with time as a fixed effect and a random intercept for patients.
HRQoL assessment
HRQoL at baseline
At baseline, patient-reported global health status (mean score, 72.2), physical functioning (mean score, 79.7), role functioning (mean score, 71.0), emotional functioning (mean score, 78.2), cognitive functioning (mean score, 87.7), and social functioning (mean score, 84.4) were moderate-to-high, reflecting moderate-to-high global health and overall functioning. Patients reported mild levels of fatigue (mean score, 34.5) and dyspnea (mean score, 30.7). The scores of the remaining symptom scales reflected low symptom severity. Similarly, patient-reported scores on the EORTC QLQ-CLL17 were low-to-mild for symptom burden (mean score, 18.0), physical condition or fatigue (mean score, 29.9), worries or fears related to health and functioning (mean score, 29.4), reflecting low-to-mild CLL-related symptoms (Table 2).
HRQoL scores and thresholds for MIDs
| Questionnaire . | Subscales . | T0 . | T1 . | T2 . | T3 . | Ptrend . | ||
|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | MID∗ . | ||||||
| EORTC QLQ-30 | Global health status | 72.2 | 23.4 | 5.9 | 83.3 | 83.1 | 82.8 | <.001† |
| Physical functioning | 79.7 | 19.6 | 8.6 | 88.9 | 89.6 | 88.4 | <.001† | |
| Role functioning | 71.0 | 35.1 | 10.9 | 89.2 | 90.5 | 88.2 | <.001† | |
| Emotional functioning | 78.2 | 20.0 | 7.84 | 88.5 | 87.0 | 86.9 | <.001† | |
| Cognitive functioning | 87.7 | 19.6 | 11.1 | 87.3 | 89.4 | 87.5 | .780 | |
| Social functioning | 84.4 | 22.4 | 12.7 | 89.4 | 93.1 | 91.3 | .004 | |
| Fatigue | 34.6 | 28.7 | 10.3 | 19.1 | 16.9 | 13.1 | <.001† | |
| Nausea and vomiting | 2.86 | 7.60 | 5.26 | 4.76 | 2.30 | 1.14 | .265 | |
| Pain | 16.9 | 25.8 | 12.8 | 9.79 | 6.32 | 7.95 | <.001 | |
| Dyspnea | 30.7 | 35.3 | 17.6 | 12.7 | 12.1 | 13.6 | <.001† | |
| Insomnia | 24.5 | 28.0 | 14.0 | 14.8 | 19.0 | 18.2 | .075 | |
| Appetite loss | 16.2 | 29.1 | 14.6 | 7.41 | 5.75 | 3.03 | <.001 | |
| Constipation | 8.85 | 21.6 | 10.8 | 6.88 | 8.05 | 10.9 | .727 | |
| Diarrhea | 11.5 | 20.8 | 10.4 | 14.2 | 7.47 | 4.65 | .036 | |
| Financial difficulties | 3.13 | 12.9 | 6.45 | 0.53 | 0.57 | 0.78 | .094 | |
| EORTC QLQ-CLL17 | Symptom burden | 18.0 | 16.8 | 9.87 | 9.10 | 8.60 | 12.2 | <.001 |
| Physical condition/fatigue | 30.0 | 26.4 | 10.8 | 13.8 | 14.5 | 11.6 | <.001† | |
| Worriers/fears | 29.4 | 18.4 | 8.70 | 17.3 | 15.4 | 19.4 | <.001† | |
| Questionnaire . | Subscales . | T0 . | T1 . | T2 . | T3 . | Ptrend . | ||
|---|---|---|---|---|---|---|---|---|
| Mean . | SD . | MID∗ . | ||||||
| EORTC QLQ-30 | Global health status | 72.2 | 23.4 | 5.9 | 83.3 | 83.1 | 82.8 | <.001† |
| Physical functioning | 79.7 | 19.6 | 8.6 | 88.9 | 89.6 | 88.4 | <.001† | |
| Role functioning | 71.0 | 35.1 | 10.9 | 89.2 | 90.5 | 88.2 | <.001† | |
| Emotional functioning | 78.2 | 20.0 | 7.84 | 88.5 | 87.0 | 86.9 | <.001† | |
| Cognitive functioning | 87.7 | 19.6 | 11.1 | 87.3 | 89.4 | 87.5 | .780 | |
| Social functioning | 84.4 | 22.4 | 12.7 | 89.4 | 93.1 | 91.3 | .004 | |
| Fatigue | 34.6 | 28.7 | 10.3 | 19.1 | 16.9 | 13.1 | <.001† | |
| Nausea and vomiting | 2.86 | 7.60 | 5.26 | 4.76 | 2.30 | 1.14 | .265 | |
| Pain | 16.9 | 25.8 | 12.8 | 9.79 | 6.32 | 7.95 | <.001 | |
| Dyspnea | 30.7 | 35.3 | 17.6 | 12.7 | 12.1 | 13.6 | <.001† | |
| Insomnia | 24.5 | 28.0 | 14.0 | 14.8 | 19.0 | 18.2 | .075 | |
| Appetite loss | 16.2 | 29.1 | 14.6 | 7.41 | 5.75 | 3.03 | <.001 | |
| Constipation | 8.85 | 21.6 | 10.8 | 6.88 | 8.05 | 10.9 | .727 | |
| Diarrhea | 11.5 | 20.8 | 10.4 | 14.2 | 7.47 | 4.65 | .036 | |
| Financial difficulties | 3.13 | 12.9 | 6.45 | 0.53 | 0.57 | 0.78 | .094 | |
| EORTC QLQ-CLL17 | Symptom burden | 18.0 | 16.8 | 9.87 | 9.10 | 8.60 | 12.2 | <.001 |
| Physical condition/fatigue | 30.0 | 26.4 | 10.8 | 13.8 | 14.5 | 11.6 | <.001† | |
| Worriers/fears | 29.4 | 18.4 | 8.70 | 17.3 | 15.4 | 19.4 | <.001† | |
SD, standard deviation; T, timepoint.
MID values are based on either the SD of the baseline score (ie, T0) for single-item scales, or on the SD of the baseline score times the square root of (1-Cronbach alpha) for multiscale items depicted in bold.
P values represent a significant change over time as well as a change from baseline that is above the MID value at least at 1 timepoint.
Longitudinal changes in HRQoL
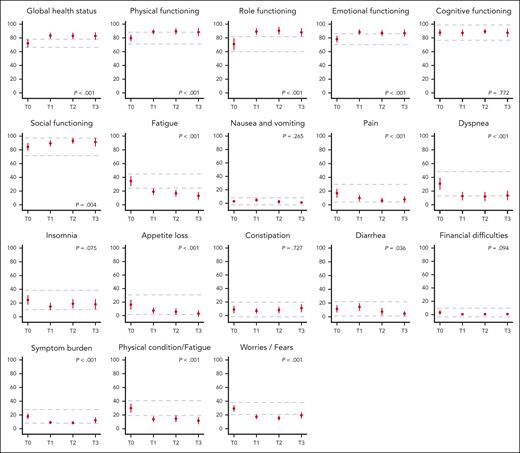
A significant improvement in HRQoL over time was observed for the majority of the subscales. Mean global health status (+10.6), physical (+8.70), role (+17.2) and emotional functioning (+8.7), fatigue (−21.5), dyspnea (−17.1), physical condition or fatigue (−18.4), and worries or fears related to health and functioning improved significantly from T0 to T3 and were considered to be clinically relevant, ie, reaching the threshold for MID (Ptrend < .001; Table 2; Figure 3; supplemental Methods). Overall, these HRQoL changes corresponded to a medium clinical effect, except for fatigue, which corresponded to a large effect size (supplemental Table 5). In general, clinically relevant improvements occurred during fixed-duration treatment (ie, T0-T1) and were sustained during consolidation treatment and follow-up (ie, T1-T3). Patients reported statistically significant, but not clinically meaningful, improvements in social functioning (+6.90; P = .004; medium effect according to Cocks et al), pain (−8.95; P < .001; small effect according to Cocks et al), and appetite loss (−13.17; P < .001; small effect according to Cocks et al; Figure 2; supplemental Table 5).
HRQoL at baseline and during the course of the treatment. Mean scores and 95% confidence intervals (vertical lines) of all the HRQoL subscales are given at baseline (T0), after 12 induction cycles (T1), 15 months after randomization (T2) and 12 months after completion of protocol treatment (T3). For functional subscales, a higher score represents a better HRQoL, for symptom subscales a higher score represents more symptoms. The dotted horizontal lines represent the calculated threshold for MID (Table 2). The P value represents the significance level of the change in HRQoL over time (supplemental Methods).
HRQoL at baseline and during the course of the treatment. Mean scores and 95% confidence intervals (vertical lines) of all the HRQoL subscales are given at baseline (T0), after 12 induction cycles (T1), 15 months after randomization (T2) and 12 months after completion of protocol treatment (T3). For functional subscales, a higher score represents a better HRQoL, for symptom subscales a higher score represents more symptoms. The dotted horizontal lines represent the calculated threshold for MID (Table 2). The P value represents the significance level of the change in HRQoL over time (supplemental Methods).
Clinically meaningful changes in HRQoL
Clinically relevant improvements were more prominent than deteriorations in the majority of the HRQoL subscales (supplemental Figure 6). The percentage of patients with improvements in functional scales increased for global health status (maximum increase of 28% from T0; P < .001), physical (+29%; P < .001), role (+23%; P < .001), emotional (+30%; P = .004), and social functioning (+24%; P = .004). The percentage of patients who showed clinically relevant improvement in symptom scales increased for fatigue (+36%; P < .001), dyspnea (+28%; P < .001), appetite loss (+22%; P < .001), symptom burden (+17%; P = .003), physical condition or fatigue (+27%; P < .001), and worries or fears related to health and functioning (+36%; P = .003; supplemental Table 6). The improvements that reached clinical relevance were comparable, irrespective of whether patients were treated with either 12 cycles of venetoclax consolidation or MRD-guided venetoclax consolidation (data not shown).
Changes in the HRQoL between consolidation arms
The improvements in HRQoL over time were statistically comparable between patients who received 12 cycles of venetoclax consolidation and MRD-guided venetoclax consolidation (supplemental Table 7). Clinically meaningful differences (ie, score difference >5 points) between the arms occurred for dyspnea (mean score, 8.89 vs 16.0) and emotional functioning at T2 (mean score, 89.4 vs 83.8). At T3, the differences between the arms in dyspnea (mean score, 10.61 vs 15.9), symptom burden (mean score, 9.09 vs 15.5), and global health status were considered to be clinically relevant (mean score, 86.4 vs 79.8 for patients treated with 12 cycles of venetoclax consolidation and MRD-guided venetoclax consolidation, respectively; supplemental Table 7).
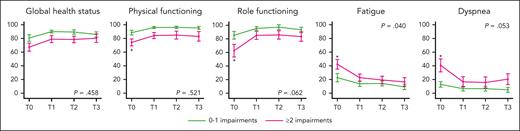
HRQoL stratified based on geriatric impairments
Overall, patients with ≥2 geriatric impairments at baseline had a lower global health status (P = .001), physical functioning (P < .001), and role functioning (P = .002) as well as more fatigue (P = .004) and dyspnea (P = .003) than patients with 0 or 1 geriatric impairments (Figure 4; supplemental Table 8). However, irrespective of the number of geriatric impairments at baseline, HRQoL improved over time in all subscales (Figure 4). The magnitude of the improvement in role functioning, fatigue, and dyspnea tended to be higher for patients with ≥2 geriatric impairments, leading to scores comparable with those of patients with 0 or 1 geriatric impairments after completing induction treatment (Figure 4). In the remaining HRQoL subscales, the number of geriatric impairments at baseline did not influence the overall scores as well as the change in HRQoL over time (supplemental Figure 7; supplemental Table 8).
HRQoL for patients with 0 or 1 and ≥2 geriatric impairments at baseline. Mean scores and 95% confidence intervals (vertical lines) are given for subscales that show differences according to the number of geriatric impairments at baseline (T0), after 12 induction cycles (T1), 15 months after randomization (T2), and 12 months after completion of protocol treatment (T3). The star (∗) represents cross-sectional significant differences (P < .005) for patients with 0 to 1 and ≥2 geriatric impairments. The P value represents the two-way interaction between the number of geriatric impairments and time. The P values for time and the number of geriatric impairments are depicted in supplemental Table 7. The remaining subscales are depicted in supplemental Figure 7.
HRQoL for patients with 0 or 1 and ≥2 geriatric impairments at baseline. Mean scores and 95% confidence intervals (vertical lines) are given for subscales that show differences according to the number of geriatric impairments at baseline (T0), after 12 induction cycles (T1), 15 months after randomization (T2), and 12 months after completion of protocol treatment (T3). The star (∗) represents cross-sectional significant differences (P < .005) for patients with 0 to 1 and ≥2 geriatric impairments. The P value represents the two-way interaction between the number of geriatric impairments and time. The P values for time and the number of geriatric impairments are depicted in supplemental Table 7. The remaining subscales are depicted in supplemental Figure 7.
Discussion
Not only cancer itself but also the various therapeutics used to combat the disease can lead to a wide range of symptoms and side effects that negatively influence the patient’s functioning and their overall QoL. Traditionally, outcomes such as the overall response rate, PFS, and OS have been used to assess the efficacy for cancer drugs. However, these clinical outcomes fail to capture the viewpoint of the patient. Particularly, when treatment does not have a curative potential, the quality and quantity of responses may not represent patient-specific relevant outcomes.46,47 Consequently, to evaluate the effectiveness of treatment modalities, especially in chronic diseases, such as CLL, it is critical to obtain comprehensive self-reported information on the patients’ symptom severity and their subjective experience of the disease and its treatment.48-50 Although the International Society of Geriatric Oncology recommends the collection of such PRO measures in patients with CLL, the availability of self-reported data in CLL remains limited.8 In this study, we report that novel fixed-duration frontline treatment for patients with CLL who are considered to be unfit for fludarabine-based therapies, improves the overall PROs and functional assessments, including the GA, the global health status as well as key symptoms and aspects of functioning.
In the HOVON139/GiVe trial, patients had a median number of 2 geriatric impairments at baseline, with gait speed, nutrition and comorbidities being the most frequently impaired domains. We found that patients with ≥2 geriatric impairments at baseline experienced more grade 3 or 4 nonhematological toxicities than patients with 0 or 1 geriatric impairment. Previous studies that performed GA in hematological malignancies also demonstrated an association between geriatric impairments and a higher cumulative incidence of nonhematological toxicities, highlighting the importance of performing a GA before therapy to identify patients with less reserves who are at risk for treatment-related toxicities.51-53 To date, only 2 studies thoroughly examined GA in older patients with CLL treated with either low-dose fludarabine with or without additional administration of erythropoiesis-stimulating agent darbepoetin alfa in the CLL9 study, or a wide range of therapies, of which chlorambucil monotherapy (57%) was mostly administrated within a single-center experience.5,6 The CLL9 study reported an association with an impaired IADL status and the occurrence of infections and reported inferior OS for patients with impairments in physical or cognitive functioning.6 However, because both these studies did not include patients treated with chemoimmunotherapy or novel targeted approaches, it cannot be extrapolated to the contemporary practices. More recently, the Alliance trial reported on baseline geriatric characterized in previously untreated patients with CLL treated with bendamustine plus rituximab, rituximab-ibrutinib, and R-ibrutinib but did not provide longitudinal data or correlations between geriatric impairments and disease-specific outcomes.9 Our study suggests comparable survival outcomes of patients with 0 or 1 and ≥2 geriatric impairments at baseline. Importantly, patients had an overall reduction in the number of geriatric impairments over time, highlighting that first-line treatment with fixed-duration Ven-O is effective and improves the overall well-being of older, unfit patients with CLL independent of the presence of geriatric impairments at baseline. Therefore, this treatment regimen should be accessible for older patients with CLL and should not be automatically withheld from the patients who are frail.
Patients with CLL have an inferior HRQoL than healthy controls and patients with other malignancies.54-56 It has been shown that chemoimmunotherapy and targeted treatment with Bruton tyrosine kinase inhibitors, phosphoinositide 3-kinase inhibitors, and venetoclax monotherapy can improve HRQoL in patients with previously untreated and relapsed or refractory CLL.16,57-63 Recently, the CLL14 trial demonstrated that 12 cycles of fixed-duration Ven-O is associated with clinically relevant improvements in the global health status (defined as an increase of 8 points), fatigue, and insomnia (defined as a decrease of 9 points).64 In this study, we demonstrated an improvement in virtually all HRQoL subscales, using more robust thresholds for the determination of clinical relevance according to the comprehensively established distribution-based and anchor-based MIDs. More specifically, we reported clinically relevant improvements for global health status, physical, role and emotional functioning, fatigue, dyspnea, physical condition or fatigue, and worries or fears related to health and functioning. Although patients did not report a clinically relevant improvement in insomnia, the magnitude of the clinical effect size according to Cocks et al was classified as medium. Because the anchor-based MIDs are based on the expert opinion of health care professionals who work with patients with cancer and use these questionnaires on a regular basis rather than a simple calculation, these MIDs might be more relevant to the patients.40,41 Consequently, for future research we recommend the use of anchor-based MIDs complementary to distribution-based MIDs.
In general, a clinically relevant improvement in HRQoL was reached after induction treatment with 12 cycles of Ven-O, which was maintained during consolidation treatment and follow-up. Although patients who were randomly assigned to receive 12 additional cycles of venetoclax as consolidation treatment experienced prolonged toxicities compared with patients who received MRD-guided venetoclax consolidation, this was not reflected by a deterioration in HRQoL.26 Strikingly, clinically meaningful differences for emotional functioning, dyspnea, symptom burden, and the global health status were reported in favor of 12 cycles of venetoclax consolidation compared with MRD-guided venetoclax consolidation. Because disease control according to MRD response, subsequent disease relapse, and survival was comparable between the arms, this observation might be explained by statistical variability or point toward physiological distress and the fear of disease relapse during a treatment-free period and could potentially be an unmet supportive care need that emerges when stopping therapy.65
The strength of our study is the use of longitudinal comprehensive PRO and functional assessment data from a clinical trial, which provide important insights into the patient experiences during the course of the treatment. Moreover, to our knowledge, this is the first trial in which GA was performed in patients with CLL treated with novel approaches and reported alongside with HRQoL data. In addition, using distribution- and anchor-based MIDs we reported tailored and patients-centered cut-off values for clinical relevance, which have not been previously reported in the context of CLL. The limitations pertain the moderate size, the lack of a comparative arm including another modern treatment regimens, such as continuous ibrutinib or chemoimmunotherapy to enable direct comparison of PROs and the exclusion of patients with ECOG performance score between 3 and 4. Because of the inclusion of patients with predominantly ECOG performance scores between 0 and 1 (96%), patients were of intermediate age (range 57-89 years) and did not have a heavy burden of geriatric impairments or features displaying phenotypic frailty, such as IADL (6%) and ADL (1%) incapabilities, which may limit the generalizability of our findings to the broader population of patients with CLL, particularly those who are frail. Although the results of our study should be interpreted carefully, our study shows that even in relatively fit patients with CLL, the GA can detect geriatric impairments and can aid in identifying patients with less reserves who are at risk for experiencing more toxicities.
In conclusion, we demonstrated that the high efficacy of Ven-O was accompanied by s reduction in geriatric impairments and an improvement in patients’ functioning and overall QoL.
Acknowledgments
The authors thank all the patients, their families, nurses, and physicians for their participation in this trial. In addition, the authors thank all the nurses who were involved in the conductance of the geriatric assessment and/or assistance of the patients with the QoL questionnaires. The authors thank Lotte Boone for performing muscle mass and muscle density measurements on the computed tomography as a second assessor.
This study was funded by F. Hoffmann-La Roche (ML29995).
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Authorship
Contribution: L.v.d.S., S.K., A.P.K., and M.-D.L. designed the study; S.K., J.D., K.N., M.-D.L., and A.P.K. accessed and verified the masked data and confirmed its accuracy and completeness; J.A.D., C.H.M.M., and A.-M.F.v.d.K.-K. did the central laboratory assessment; J.D. performed the MRD analysis; L.v.d.S. analyzed the data and wrote the first draft of the manuscript; and all authors critically reviewed the data and read, commented, and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.P.K. reports personal fees from AbbVie, LAVA, Genmab, Janssen, AstraZeneca, Roche/Genentech, and Bristol Myers Squibb and research funding from AbbVie, Janssen, AstraZeneca, Roche/Genentech, and Bristol Myers Squibb. A.W.L. reports research funding from Roche/Genentech, AbbVie, Gilead, and Janssen and speakers fee from Janssen and Gilead. E.v.d.S. reports honoraria from Janssen and Amgen and support for attending meetings from Janssen. J.D. reports research funding from Roche/Genentech. S.K. reports personal fees from Janssen, AbbVie, Novartis, Gilead, and Celgene; and research funding from AbbVie, Janssen, AstraZeneca, and Roche/Genentech. M.-D.L. reports personal fees from AbbVie, Janssen, and Roche and research funding from AbbVie, Janssen, AstraZeneca, and Roche/Genentech. S.H.T. reports personal fees from Roche, Takeda, Incyte, Kite/Gilead, and Celgene. The remaining authors declare no competing financial interests.
A complete list of the members of the the HOVON CLL working group appears in the supplemental Appendix.
Correspondence: Mark-David Levin, Department of Internal Medicine, Albert Schweitzer Hospital, Albert Schweitzerplaats 25, 3318 AT Dordrecht, The Netherlands; e-mail: m-d.levin@asz.nl.
References
Author notes
Deidentified patient data are available on request from the corresponding author, Mark-David Levin (m-d.levin@asz.nl). The HOVON CLL working group and Roche will evaluate these requests on a case-by-case basis.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal