TO THE EDITOR:
Erdheim-Chester disease (ECD) is a rare, systemic histiocytic disorder characterized by tissue infiltration by foamy CD68+ CD1a− histiocytes.1 Its clinical presentation ranges from a localized condition to a multisystem and life-threatening disease.2 Patients with ECD have significantly benefited from the recent introduction of targeted therapies (ie, BRAF inhibitors [BRAFis], MEK inhibitors [MEKis], and mammalian target of rapamycin inhibitors),3-5 but some still have a refractory course or experience long-term disability.6
ECD typically occurs in adulthood, and data on pediatric cases have so far been sporadic. Therefore, the most appropriate diagnostic and therapeutic approach in children remains uncertain. An additional challenge in diagnosing pediatric ECD resides in the pathologic similarity with juvenile xanthogranuloma (JXG), which displays similar morphology and immunostaining features.2,7 However, JXG is usually a localized cutaneous disease, and its systemic form lacks most of the clinical and mutational characteristics of ECD.8-10
Through a large collaboration involving international networks of centers with expertise in histiocytic disorders, we collected 21 cases of childhood-onset ECD that were managed in the past decade, after the introduction of targeted therapies. We screened patients with biopsy-proven ECD or mixed ECD/Langerhans cell histiocytosis (LCH)1,11 and then included patients who fulfilled the following criteria: (1) age at ECD onset <18 years; (2) clinical presentation consistent with ECD with at least 1 typical localization (long-bone involvement, perirenal infiltration, large-vessel infiltration, cardiac involvement, or xanthelasma; see supplemental Methods [available on the Blood website]); (3) available data on molecular studies (at least BRAF mutation determination); and (4) available data on treatment and outcome with a minimum follow-up of 6 months. These criteria were selected to better discriminate pediatric ECD from other conditions with similar pathology, such as JXG and LCH with xanthomatized bone lesions. Response to treatment was a composite end point, which included clinical, radiologic,12 and metabolic13 response criteria, as detailed in the supplemental Methods. Organ involvement definition and molecular studies are also reported in the supplemental Methods.
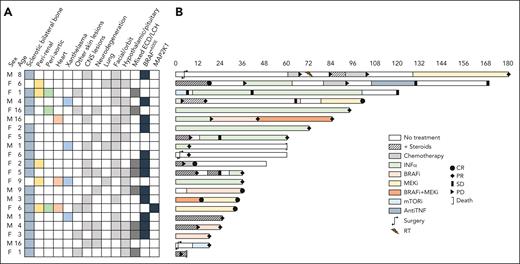
Twenty-eight patients with childhood-onset ECD were screened, and 21 were finally included (5 were not eligible, and 2 had insufficient follow-up; supplemental Figure 1); 4 of them (patients 9, 10, 11, and 13) were previously published cases.14-17 The patients’ baseline characteristics are shown in Figure 1 and in supplemental Table 1. Twelve patients (57%) were female; the median age at symptom onset and diagnosis was 5 (interquartile range [IQR], 2-8) and 6 (IQR, 3.5-11.5) years, respectively. The most frequent symptoms at presentation were systemic (eg, fatigue or fever; n = 14 [67%]), polyuria/polydipsia (n = 9 [43%]), bone pain (n = 7 [33%]), and neurologic deficits (n = 5 [24%]) (supplemental Table 1). The most frequently involved organs were the bone (n = 18 [86%]), the facial/orbital structures (n = 14 [67%]), the central nervous system (CNS) (n = 13 [62%], 9 with pseudotumoral masses and 7 with infiltrative/atrophic lesions), the hypothalamic/pituitary axis (n = 12 [57%]), the skin (n = 9 [43%], 4 had xanthelasmas), the perirenal tissue (n = 5 [24%]), and, more rarely, the aorta, the heart, and the lungs (3 cases each [14%]) (Figures 1-2 and supplemental Table 1). Typical pathologic findings are reported in Figure 2. Twelve patients (57%) had the BRAFV600E mutation, and 1 an MAP2K1 mutation. Patients harboring the BRAFV600E mutation presented more frequently with exophthalmos (P = .007) (supplemental Table 2). Eight patients (38%) had mixed ECD/LCH and showed a significantly higher frequency of systemic symptoms (P = .007), diabetes insipidus (P = .032), and pituitary insufficiency (P = .023) than patients with isolated ECD (supplemental Table 3). Compared with aggregated data from previously reported cases (referenced in supplemental Table 4), our patients had a younger age at disease onset, more frequent multisystem (≥4 affected sites) involvement, higher rates of skin and facial/orbit involvement, and a higher rate of pituitary insufficiency (supplemental Table 1). When we compared our series with 3 large series of adult patients with ECD,6,18,19 we found more frequent CNS and facial/orbit involvement, and a higher proportion of mixed ECD/LCH; conversely, typical features of adult ECD, such as perirenal, large-vessel, pulmonary, and cardiac lesions, were less common in our cohort (supplemental Figure 2).
Demographics and clinical presentation (A) and swimmer plot of treatment and outcome (B) in the 21 patients included in the study. AntiTNF, anti–tumor necrosis factor; CNS, central nervous system; CR, complete response; INF-α, interferon-alfa; mTORi, mammalian target of rapamycin inhibitor; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease.
Demographics and clinical presentation (A) and swimmer plot of treatment and outcome (B) in the 21 patients included in the study. AntiTNF, anti–tumor necrosis factor; CNS, central nervous system; CR, complete response; INF-α, interferon-alfa; mTORi, mammalian target of rapamycin inhibitor; PD, progressive disease; PR, partial response; RT, radiotherapy; SD, stable disease.
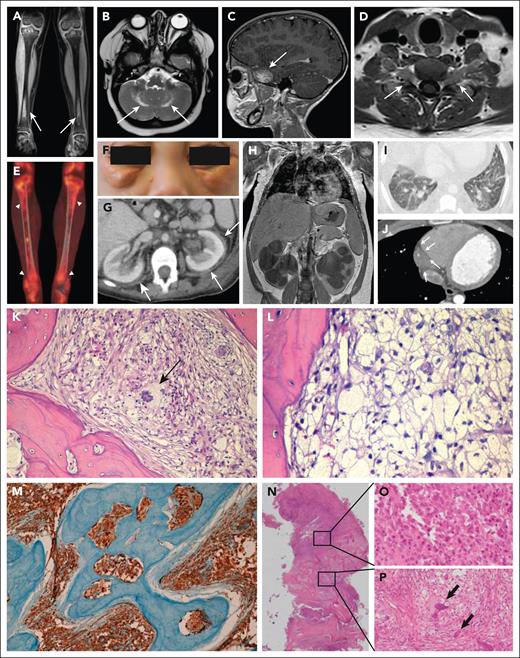
Typical ECD manifestations and bone pathology in patients with ECD and mixed ECD-LCH. (A) Coronal magnetic resonance imaging (MRI) scan showing bilateral symmetric osteosclerosis of tibial meta-diaphysis (arrows). (B) Axial MRI scan of the brain showing bilateral hyperintense lesions of the dentate nuclei (arrows). (C) Sagittal MRI scan of the brain depicting a skull base meningioma-like lesion of the greater sphenoid wing (arrow). (D) Axial MRI scan of the cervical spine showing a bilateral lesion with slight enhancement forming a dumbbell-shaped mass inside the neural foramina at C5-C6 level (arrows). (E) 18F-fluorodeoxyglucose positron emission tomography showing high metabolic activity of tibial metadiaphyseal lesions (arrowheads). (F) Periorbital xanthelasma-like lesions. (G) Axial abdominal computed tomography (CT) scan showing bilateral perinephric infiltrates (“hairy kidneys”) (arrows). (H) Coronal abdominal CT scan showing massive bilateral hydroureteronephrosis in a patient with perinephric ECD infiltrates. (I) High-resolution axial CT scan of the chest demonstrating bilateral interlobular septal thickening and ground-glass opacities. (J) Axial CT scan of the chest showing cardiac pseudotumor (arrows) developed around the right coronary artery and into the right atrioventricular sulcus. (K) Bone biopsy showing an infiltrate comprising numerous foamy histiocytes and a multinucleated Touton cell (arrow) associated with fibrosis. (L) Diffuse infiltration by foamy histiocytes. (M) Classic ECD CD68 immunostaining in a bone biopsy. (N) Mixed lesion showing an area occupied by numerous Langerhans cells with ovoid to reniform nuclei, consistent with LCH (O) and a concomitant area showing numerous foamy histiocytes and multinucleated Touton cells (arrows), consistent with ECD (P). K-L and N-P: Hematoxylin and eosin staining; M: immunohistochemical staining for CD68-PGM1. Original magnification: K, ×20; L, ×40; M, ×10; N, ×2; O-P, ×40.
Typical ECD manifestations and bone pathology in patients with ECD and mixed ECD-LCH. (A) Coronal magnetic resonance imaging (MRI) scan showing bilateral symmetric osteosclerosis of tibial meta-diaphysis (arrows). (B) Axial MRI scan of the brain showing bilateral hyperintense lesions of the dentate nuclei (arrows). (C) Sagittal MRI scan of the brain depicting a skull base meningioma-like lesion of the greater sphenoid wing (arrow). (D) Axial MRI scan of the cervical spine showing a bilateral lesion with slight enhancement forming a dumbbell-shaped mass inside the neural foramina at C5-C6 level (arrows). (E) 18F-fluorodeoxyglucose positron emission tomography showing high metabolic activity of tibial metadiaphyseal lesions (arrowheads). (F) Periorbital xanthelasma-like lesions. (G) Axial abdominal computed tomography (CT) scan showing bilateral perinephric infiltrates (“hairy kidneys”) (arrows). (H) Coronal abdominal CT scan showing massive bilateral hydroureteronephrosis in a patient with perinephric ECD infiltrates. (I) High-resolution axial CT scan of the chest demonstrating bilateral interlobular septal thickening and ground-glass opacities. (J) Axial CT scan of the chest showing cardiac pseudotumor (arrows) developed around the right coronary artery and into the right atrioventricular sulcus. (K) Bone biopsy showing an infiltrate comprising numerous foamy histiocytes and a multinucleated Touton cell (arrow) associated with fibrosis. (L) Diffuse infiltration by foamy histiocytes. (M) Classic ECD CD68 immunostaining in a bone biopsy. (N) Mixed lesion showing an area occupied by numerous Langerhans cells with ovoid to reniform nuclei, consistent with LCH (O) and a concomitant area showing numerous foamy histiocytes and multinucleated Touton cells (arrows), consistent with ECD (P). K-L and N-P: Hematoxylin and eosin staining; M: immunohistochemical staining for CD68-PGM1. Original magnification: K, ×20; L, ×40; M, ×10; N, ×2; O-P, ×40.
First-line medical therapies included chemotherapy in 8 patients (38%; in 7, according to the LCH-IV protocol; NCT02205762), interferon-alfa in 6 (29%), and targeted therapies in 6 (29%); 1 patient (patient 10) did not receive medical treatment because his symptoms (bone pain) spontaneously resolved (Figure 1). Ten patients (48%) required >1 line of treatment for disease progression or toxicity: 5 of them received BRAFi or MEKi, 4 received interferon-alfa, 3 underwent chemotherapy (cladribine, methotrexate, and vinblastine, respectively), and 1 received anti–tumor necrosis factor agents; corticosteroids and radiotherapy were also used in 2 cases to mitigate symptoms. Best objective responses were observed in the 8 patients receiving BRAFi and MEKi (complete responses and partial responses [PRs] in 4 cases each) and in those receiving interferon-alfa (PR in 5 of 8 treated patients). The efficacy of chemotherapy was limited (only 2 sustained responses of 8 treated patients). After a median follow-up of 49 months (IQR, 25-84 months), 2 patients died: patient 9 received interferon-alfa and achieved a PR, but treatment was discontinued for financial reasons; the patient was lost to follow-up and died 5 years after diagnosis under unspecified circumstances; patient 21 received chemotherapy (LCH-IV; NCT02205762) but progressed and died of treatment-related septic shock. The remaining 19 patients had either stable disease (2 patients) or response (17 patients; complete response in 5 and PR in 12) at last follow-up (Figure 1).
The treatments were only partially tolerated: grade 2 or higher adverse effects were reported in 10 patients (48%), requiring discontinuation in 8. Of note, 50% of patients receiving BRAFi/MEKi had at least grade 2 toxicities, but adverse effects were well controlled by dose reduction, except for 2 cases that required BRAFi discontinuation for ocular toxicity (supplemental Table 5). Long-term disabilities were also common (n = 16 [76%]), mostly involving the endocrine system (diabetes insipidus); renal, ocular, and neuropsychiatric sequelae were reported as well (supplemental Table 6).
This is the first series of pediatric ECD that provides detailed clinical and molecular patient characterization. The mutational profile of our pediatric cohort was similar to that of adult ECD, with ≈60% of patients harboring the BRAFV600E mutation, while other mutations, such as MAP2K1, were less frequently detected. Features such as bone and skin lesions occurred in children with a frequency similar to that seen in adults, whereas other typical manifestations of adult ECD (ie, perirenal, periaortic, and cardiac) were less frequent in children. Conversely, sites more commonly involved in children than in adults were the facial/orbital structures, the CNS, and the hypothalamic/pituitary system; mixed ECD/LCH was also more frequent in children, a finding in keeping with the higher prevalence of LCH in childhood.
The management of children with ECD is challenging, considering its multisystem involvement, its long-term nature, and patients’ age. In this study, pediatric ECD reflected much of the complexity of adult ECD and presented in many cases as a difficult-to-treat disease. The treatments administered to our patients were varied, but an overall pattern of response emerged. Most patients were started on regimens borrowed from LCH, which were rarely effective, and required rescue treatments. Similar to adults,20 interferon-alfa proved effective in children, although careful monitoring of adverse effects is essential. With respect to targeted molecules (BRAFi and MEKi), our data reinforce the view that these treatments also work in children, as they showed rapid efficacy both as first-line options and in patients refractory to other therapies. These agents have an acceptable tolerability profile in children, but the questions regarding treatment discontinuation after response and potential long-term toxicity remain.
Acknowledgments
This work was supported in part by National Institutes of Health, National Cancer Institute (NCI) Cancer Center Support grant P30 CA008748 (E.L.D., I.J.D.), NCI grant R37CA259260 (E.L.D.), the Frame Family Fund (E.L.D.), the Joy Family West Foundation (E.L.D.), the Applebaum Foundation (E.L.D.), and the Erdheim-Chester Disease Global Alliance (E.L.D).
Authorship
Contribution: F.P. and A.V. conceived the study, collected data, and drafted the manuscript; M.M., J.P., J.D., J.-F.E., E.S., and J.H. contributed to designing the manuscript, participated in its preparation, and critically revised it; F.P., M.M., I.T., S.B., S.M., I.J.D., C.v.d.B., Ö.T., S.S., S.O., F.G., C.D.F., S.G., A.M.B., M.L.C., E.B., P.R., J.D., E.L.D., J.-F.E., E.S., J.H., and A.V. followed up the patients and/or performed pathologic studies; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: I.J.D. has been a consultant for AstraZeneca, Bristol Myers Squibb, GSK, and Pyramid Bioscience. Memorial Sloan Kettering Cancer Center has received funding (for costs associated with pharma-sponsored studies on which I.J.D. served as institutional principal investigator) from Bristol Myers Squibb, Genentech, and Novartis. E.L.D. discloses unpaid editorial support from Pfizer and serves on an advisory board for Day One Therapeutics, Opna Bio, and Springworks Therapeutics, both outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Augusto Vaglio, Dipartimento di Scienze Biomediche, Sperimentali e Cliniche “Mario Serio,” Università di Firenze, Viale Pieraccini 6, 50139 Firenze, Italy; e-mail: augusto.vaglio@unifi.it.
References
Author notes
∗E.S., J.H., and A.V. share senior authorship.
Data will be shared on contact with the corresponding author via email.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal