TO THE EDITOR:
B-cell maturation antigen (BCMA)-targeting chimeric antigen receptor (CAR) T cells have revolutionized the treatment for patients with relapsed and refractory multiple myeloma, with 2 US Food and Drug Administration–approved products, idecabtagene vicleucel and ciltacabtagene autoleucel (cilta-cel).1,2 Follow-up of patients receiving cilta-cel in the CARTITUDE-1 study found 82.5% of patients achieved a stringent complete response,3 with a median progression-free survival of 34.9 months.4 However, in addition to common complications, like cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), and prolonged cytopenia seen with other CAR products,5-9 movement and neurocognitive toxicities (MNTs) have also developed in patients receiving BCMA-targeting CARs.1,3,10 MNTs occurred in 6% of patients treated in CARTITUDE-1, but they have been less frequent in the more recent cilta-cel trials.3,11 Risk factors for MNTs include high CAR expansion, high tumor burden, any grade ICANS, and grade ≥3 CRS.10 No data are currently available on the incidence of MNTs in the postapproval era, and literature guiding clinicians on how to manage this complication is limited. Only a single detailed case report is available describing a fatal outcome, which was thought to be attributable to on-target, off-tumor toxicity of CAR T cells infiltrating the basal ganglia.12 Here, we report the successful treatment of severe MNTs, associated with massive CAR expansion, and investigate the characteristics of persisting CAR T cells.
A 74-year-old man with relapsed IgA λ multiple myeloma received cilta-cel as fifth-line therapy. He was diagnosed 3.5 years previously with International Staging System stage IIIA disease, and cytogenetics showed t(11;14) and 1q gain. Previous therapies included daratumumab, lenalidomide, bortezomib, and dexamethasone; high-dose melphalan and autologous stem cell transplant; and carfilzomib, venetoclax, and dexamethasone. Pretreatment bone marrow biopsy demonstrated λ-restricted plasma cells comprising <5% of nucleated cells. Serum protein electrophoresis identified 2 IgA paraprotein bands totaling 0.54 g/dL, and the difference between involved and uninvolved light chains was 309.5 mg/L. Medical history included coronary artery disease, benign prostatic hypertrophy, a benign parathyroid adenoma, and osteoarthritis. He had no history of smoking or recreational drug use and drank alcohol only occasionally. He had no personal or family history of neurologic disease.
Following standard cyclophosphamide and fludarabine lymphodepletion, he was infused with 0.6 × 106/kg CAR+ T cells (cilta-cel). On day (D) +6, he developed concurrent grade 2 CRS and grade 2 ICANS with an immune effector cell (ICE) score of 3 of 10 (American Society for Transplantation and Cellular Therapy grading)13 (Figure 1A). He was managed with tocilizumab, corticosteroids, and anakinra (Figure 1B). He improved, and by D+10, anti–cytokine-directed therapy was discontinued.
Massive CAR T-cell expansion coincides with toxicity. (A) Time course of adverse events following CAR T-cell infusion. The change in color of the MNTs indicates the timing of cyclophosphamide. (B) Therapeutic strategies deployed for CAR T-cell–related toxicity. (C) Absolute lymphocyte count (ALC) and CAR T-cell count (cells/μL blood) are plotted on the left y-axis, and vector copy number (VCN) per cell is plotted on the right y-axis. Absolute CAR T-cell number decreases following cyclophosphamide therapy, but CAR T cells remain the predominant population of circulating lymphocytes. (D) M protein is plotted on the left y-axis, and the difference between involved and uninvolved serum-free light chains is plotted on the right y-axis. Both biomarkers decrease rapidly following CAR T-cell infusion and remain low for the duration of follow-up, consistent with an ongoing stringent complete response. (E) Immunophenotyping demonstrates CAR T-cell expansion, gated on CD3+, viable lymphocytes. (F) Magnetic resonance imaging (MRI) of the brain showed that the caudate, striatum, and other structures comprising the basal ganglia were normal. A prior comparison MRI of the brain was not available. (G-H) Brain (18)F-fluorodeoxyglucose–positron emission tomography images showed normal metabolic activity in the basal ganglia and occipital lobes (G) and mild bilateral hypometabolism in the frontal lobes, anterior cingulate gyri, and, to a lesser degree, parietal lobes (H). APC, allophycocyanin; DIC, disseminated intravascular coagulation; FLC, free light chains; PE, phycoerythrin; sBCMA, soluble BCMA; UTI, urinary tract infection.
Massive CAR T-cell expansion coincides with toxicity. (A) Time course of adverse events following CAR T-cell infusion. The change in color of the MNTs indicates the timing of cyclophosphamide. (B) Therapeutic strategies deployed for CAR T-cell–related toxicity. (C) Absolute lymphocyte count (ALC) and CAR T-cell count (cells/μL blood) are plotted on the left y-axis, and vector copy number (VCN) per cell is plotted on the right y-axis. Absolute CAR T-cell number decreases following cyclophosphamide therapy, but CAR T cells remain the predominant population of circulating lymphocytes. (D) M protein is plotted on the left y-axis, and the difference between involved and uninvolved serum-free light chains is plotted on the right y-axis. Both biomarkers decrease rapidly following CAR T-cell infusion and remain low for the duration of follow-up, consistent with an ongoing stringent complete response. (E) Immunophenotyping demonstrates CAR T-cell expansion, gated on CD3+, viable lymphocytes. (F) Magnetic resonance imaging (MRI) of the brain showed that the caudate, striatum, and other structures comprising the basal ganglia were normal. A prior comparison MRI of the brain was not available. (G-H) Brain (18)F-fluorodeoxyglucose–positron emission tomography images showed normal metabolic activity in the basal ganglia and occipital lobes (G) and mild bilateral hypometabolism in the frontal lobes, anterior cingulate gyri, and, to a lesser degree, parietal lobes (H). APC, allophycocyanin; DIC, disseminated intravascular coagulation; FLC, free light chains; PE, phycoerythrin; sBCMA, soluble BCMA; UTI, urinary tract infection.
His absolute lymphocyte count (ALC) climbed to 29 720 cells/μL blood on D+14 (Figure 1C), and we were able to track the frequency of CAR+ T cells in his circulation over time (Figure 1E). Immunophenotyping revealed >99% of CD3+ T cells were CAR+, and vector copy number, by droplet digital polymerase chain reaction, demonstrated similar expansion kinetics (Figure 1C). Following resolution of his short-term CAR-related toxicities, he was discharged from the hospital on D+16. Routine follow-up on D+17 identified fatigue but no other symptoms. He was readmitted on D+20 with difficulty walking and neurocognitive impairment (ICE score, 6 of 10), which his wife had noticed developing over the previous day (D+19). Neurologic examination revealed inattention, masked facies, hypophonia, intention tremor, and cogwheeling at the right wrist. He had a slow, mildly wide-based, and shuffling gait with stooped posture and decreased stride length. Light touch sensation, strength, coordination, and cranial nerves were intact throughout. There was no pronator drift. Reflexes were symmetric and normal in amplitude at the upper extremities, but hyporeflexive at the patellae and ankles. Brain magnetic resonance imaging on D+21 showed no structural abnormality or abnormal regions of restricted diffusion, contrast enhancement, or suggestion of acute or evolving structural pathologic features; there were mild scattered foci of T2/fluid-attenuated inversion recovery hyperintensity in the bilateral supratentorial white matter that were most consistent with chronic microvascular disease (Figure 1F). The patient was treated with dexamethasone, 10 mg a day, and anakinra, 100 mg twice a day, for 10 days. By D+24, he was less confused (ICE score, 10 of 10), he was more interactive, and his cogwheel rigidity had resolved, but he had residual hypomimia, hypophonia, inattention, and gait abnormalities.
His ALC remained elevated at 11 150 cells/μL blood with 99.3% of circulating T cells CAR+ on D+38. Because of concern about the potential development of irreversible neurologic damage, he was treated with 2 g/m2 IV cyclophosphamide on D+38. Within 24 hours of cyclophosphamide therapy, there was a significant improvement in his gait, psychomotor slowing, inattentiveness, and hypomimia, but he continued to have residual intention tremor. Although the total ALC decreased to 1360 cells/μL blood, 98% of his circulating T cells remained CAR+ (Figure 1E). The patient's neurologic symptoms continued to improve, and prednisone was tapered off over 3 months. At the last follow-up (D+126), MNTs had completely resolved. Interim disease response assessments, including a marrow biopsy that found no residual myeloma, and serum protein electrophoresis showed a rapid decline in IgA paraprotein, which became undetectable by D+83, and serum free light chain levels were undetectable (Figure 1D). Positron emission tomography CT on D+56 showed no myeloma lesions, consistent with a stringent complete response (International Myeloma Working Group criteria, 2016).14 Brain imaging showed normal metabolic activity in the basal ganglia and occipital lobes (Figure 1G). There was mild hypometabolism in the frontal lobes and anterior cingulate gyri of unknown significance (Figure 1H).
During his cyclophosphamide course, the patient developed low-grade fevers, hematuria, BK viruria, and a bacterial urinary tract infection with Klebsiella pneumoniae, which resolved with antimicrobials and IV immunoglobulin. Because of concerns about the massive proliferation and then chemorefractory nature of the residual CARs in the absence of detectable bone marrow plasma cells, further characterization was performed to assess T-cell clonality and additional drivers of persistence.
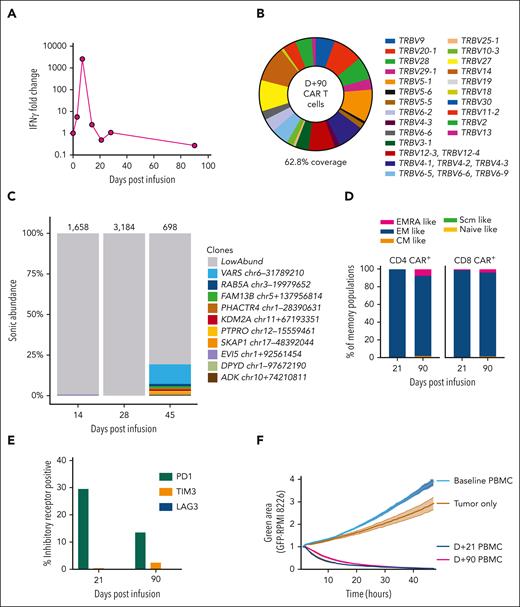
Vector copy number peaked at 1.05 copies per cell, suggesting that high transduction was not the cause of the outgrowth of CARs. A dramatic increase in serum interferon gamma, with >2500-fold increase, was observed on D+7, from a preinfusion value of 1.29 pg/mL to a peak value of 3323 pg/mL (Figure 2A). However, interferon gamma levels, along with other measured cytokines, were returning to baseline at the time of MNTs. T-cell receptor clonality testing on D+24 and D+52 samples, using polymerase chain reaction for γ chain rearrangements, did not identify a clonal population. This was consistent with flow cytometry evaluation of Vβ repertoire on D+90, which was polyclonal (Figure 2B). Integration site analysis on samples from D+14 and D+28 was polyclonal, whereas at D+45, there was a clone reaching 12% of the population with an integration site within VARS1, which encodes a highly conserved enzyme essential for mRNA translation15,16 (Figure 2C). This clone could not account for the initial CAR proliferation or the patient's symptoms but emerged following cyclophosphamide therapy.
Phenotypic and functional properties of CAR T cells. (A) Serum interferon gamma (IFN-γ) fold change from baseline is displayed. Levels increase rapidly following CAR T-cell infusion and peak at D+7 after infusion at 2576-fold increase. Baseline value was 1.29 pg/mL, increasing to 3323 pg/mL on D+7. (B) Flow cytometry assessment of T-cell receptor Vβ repertoire gated on CAR+ T cells from a D+90 peripheral blood mononuclear cell (PBMC) sample. This assay detected Vβ repertoire of 62.8% of CAR+ T cells, which was a similar level of coverage to healthy donor T cells run in parallel (data not shown). (C) The relative clonal abundance of lentiviral integration sites is displayed. Most are of low abundance (gray shading), consistent with a polyclonal population of transduced cells. At D+45, a small clone (12%) with an integration site in VARS1 emerged. Numbers above the columns indicate the numbers of cells sampled. Genomic locations of the most abundant clones (all found at D+45) are shown in the key at right (numbers refer to locations on the hg38 draft of the human genome sequence). (D) Immunophenotyping of CD4 CAR+ and CD8 CAR+ T cells from D+21 and D+90 PBMCs is shown. Naïve-like (CD45RA+CCR7+CD95−), stem cell memory (Scm)–like (CD45RA+CCR7+CD95+), central memory (CM)–like (CD45RA−CCR7+), effector memory (EM)–like (CD45RA−CCR7−), and EMRA-like (CD45RA+CCR7−) populations are displayed. Most CAR T cells are EM-like, with a small population of EMRA-like CAR T cells emerging at D+90. (E) PD1, TIM3, and LAG3 expression levels were assessed by immunophenotyping. The percentages of CAR+ T cells at D+21 and D+90 expressing these inhibitory receptors are displayed. (F) A real-time cytotoxicity assay of PBMCs from D+21 and D+90 was performed against the BCMA/green fluorescent protein (GFP)–positive target cell line RPMI-8226 (in a 1:1 effector–to–target cell ratio). The green area corresponds to viable GFP+ cells and inversely relates to cytotoxicity. Triplicates were performed, and mean and standard error of the mean are plotted. Both D+21 and D+90 PBMCs rapidly eliminated tumor cells, compared with tumor cells cultured alone or with baseline (D-5) PBMCs.
Phenotypic and functional properties of CAR T cells. (A) Serum interferon gamma (IFN-γ) fold change from baseline is displayed. Levels increase rapidly following CAR T-cell infusion and peak at D+7 after infusion at 2576-fold increase. Baseline value was 1.29 pg/mL, increasing to 3323 pg/mL on D+7. (B) Flow cytometry assessment of T-cell receptor Vβ repertoire gated on CAR+ T cells from a D+90 peripheral blood mononuclear cell (PBMC) sample. This assay detected Vβ repertoire of 62.8% of CAR+ T cells, which was a similar level of coverage to healthy donor T cells run in parallel (data not shown). (C) The relative clonal abundance of lentiviral integration sites is displayed. Most are of low abundance (gray shading), consistent with a polyclonal population of transduced cells. At D+45, a small clone (12%) with an integration site in VARS1 emerged. Numbers above the columns indicate the numbers of cells sampled. Genomic locations of the most abundant clones (all found at D+45) are shown in the key at right (numbers refer to locations on the hg38 draft of the human genome sequence). (D) Immunophenotyping of CD4 CAR+ and CD8 CAR+ T cells from D+21 and D+90 PBMCs is shown. Naïve-like (CD45RA+CCR7+CD95−), stem cell memory (Scm)–like (CD45RA+CCR7+CD95+), central memory (CM)–like (CD45RA−CCR7+), effector memory (EM)–like (CD45RA−CCR7−), and EMRA-like (CD45RA+CCR7−) populations are displayed. Most CAR T cells are EM-like, with a small population of EMRA-like CAR T cells emerging at D+90. (E) PD1, TIM3, and LAG3 expression levels were assessed by immunophenotyping. The percentages of CAR+ T cells at D+21 and D+90 expressing these inhibitory receptors are displayed. (F) A real-time cytotoxicity assay of PBMCs from D+21 and D+90 was performed against the BCMA/green fluorescent protein (GFP)–positive target cell line RPMI-8226 (in a 1:1 effector–to–target cell ratio). The green area corresponds to viable GFP+ cells and inversely relates to cytotoxicity. Triplicates were performed, and mean and standard error of the mean are plotted. Both D+21 and D+90 PBMCs rapidly eliminated tumor cells, compared with tumor cells cultured alone or with baseline (D-5) PBMCs.
Next, we assessed the phenotype and functionality of persisting CARs at D+21 and D+90. We found most CAR+ T cells were effector memory-like (CD45RA−CCR7−), with a higher proportion of effector memory cell reexpressing CD45RA (EMRA)-like (CD45RA+CCR7−) CARs at D+90 (Figure 2D). Programmed cell death protein 1 (PD1) expression was upregulated at D+21, likely attributable to increased activation, with low expression of T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3) and lymphocyte-activation gene 3 (LAG3) (Figure 2E). Importantly, both D+21 and D+90 CAR T cells isolated from the patient’s peripheral blood rapidly eliminated BCMA+ tumor cells in a real-time cytotoxicity assay (Figure 2F). These findings suggest the infused CARs in the circulation remained highly functional despite resolution of MNTs.
In a published case of MNTs, the patient was treated with cyclophosphamide, 300 mg/m2 IV, intrathecal cytarabine (100 mg), and hydrocortisone.12 However, because that patient died of sepsis, the long-term effect on neurologic symptoms and CAR persistence could not be evaluated.12 Of 5 patients who developed MNTs in CARTITUDE-1, 3 died of infection or neurotoxicity,10 and there is limited published information on the remaining patients. Treatment approaches have included high-dose methylprednisolone, plasmapheresis, and IV immunoglobulin, with stable symptoms reported.10
In our case, relatively early intervention was associated with a complete and sustained reversal of neurologic symptoms without compromising anti-tumor activity at 5 months after infusion, despite functional persistence of cilta-cel CAR T cells. The mechanism underlying rapid improvement in MNTs is unclear, and it could potentially be attributable to reduction in trafficking to the central nervous system as systemic antigen burden is reduced or possibly a threshold effect, whereby MNTs only occur when there are high levels of circulating BCMA-directed CAR T cells. Early and definitive intervention in the setting of high ALC and MNTs is a potentially useful therapeutic strategy that preserves anti-tumor effects, but future studies would be needed to confirm these findings.
Written informed consent was obtained for biobanking and collection of clinical data on an Institutional Review Board–approved protocol at the Dana-Farber/Harvard Cancer Center (DFHCC number 16-206).
Acknowledgments
The authors thank the patient and his family for participating in this study. The authors also thank Aoife Roche from the Viral Molecular High-Density Sequencing Core at the University of Pennsylvania for supporting lentiviral integration site analysis, and Hannah Nolan for the Immune Monitoring Laboratory at Massachusetts General Hospital for sample processing.
This work is supported by the 2022 AACR-Genmab Non-Hodgkin B-Cell Lymphoma Research Fellowship to C.E.G. (grant 22-40-72-GRAH) and research funding from National Institutes of Health, National Cancer Institute to M.V.M. (grant R01CA252940).
Authorship
Contribution: C.E.G., W.-H.L., H.R.W., V.M.S., M.B.L., F.B., A.P., and K.M.E.G. performed the experiments; C.E.G., W.-H.L., H.R.W., J.E., F.D.B., M.V.M., K.M.E.G., and M.J.F. analyzed the data; A.J.Y., H.S., D.C., I.A.-R., and M.J.F. reviewed the patient and provided expert opinion; C.E.G., M.V.M., K.M.E.G., and M.J.F. designed the study; C.E.G., W.-H.L., M.V.M., K.M.E.G., and M.J.F. wrote the manuscript; and all other authors read, edited, and approved the manuscript.
Conflict-of-interest disclosure: M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital (some licensed to Promab) and the University of Pennsylvania (some licensed to Novartis). M.V.M. receives grant/research support from Kite Pharma. M.V.M. has served as a consultant for multiple companies involved in cell therapies. M.V.M. holds equity in 2SeventyBio, Century Therapeutics, Neximmune, Oncternal, and TCR2; and serves on the Board of Directors of 2Seventy Bio. A.J.Y. reports consulting for AbbVie, Adaptive Biotechnologies, Amgen, BMS, Celgene, GSK Janssen, Karyopharm, Oncopeptides, Prothena, Regeneron, Sanofi, and Takeda. M.J.F. reports consulting for BMS, Novartis, Kite/Gilead, Iovance, and JnJ/Legend; and research funding from Kite/Gilead and Incyte. F.D.B. is a scientific cofounder of Biocept; has intellectual property licensed to Novartis; and is a consultant for SANA, Poseida, Encoded, Roche, and Johnson and Johnson. The remaining authors declare no competing financial interests.
Correspondence: Marcela V. Maus, Massachusetts General Hospital Cancer Center, 149 13th St, Charlestown, MA 02129; e-mail: mvmaus@mgh.harvard.edu.
References
Author notes
∗C.E.G. and W.-H.L. contributed equally to this study.
†M.V.M., M.J.F., and K.M.E.G. are joint senior authors.
For original data, please contact mvmaus@mgh.harvard.edu.
A description of a series on neurologic toxicities that includes this case is in an article by Karschnia et al17 in this issue.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal