TO THE EDITOR:
Over the past 5 decades, the combination of an anthracycline and cytarabine (“3+7”) remains the standard of care for patients with newly diagnosed acute myeloid leukemia (AML) who are suitable for intensive induction therapy.1 Complete remission (CR) can be achieved in 65% to 75% of younger patients (≤60 years) and in 40% to 60% of older patients (>60 years).2,3 However, patients in the adverse-risk category who received intensive chemotherapy (IC) had significantly lower CR rates and more dismal outcomes than those in favorable- and intermediate-risk categories based on the revised 2017 European LeukemiaNet (ELN) genetic risk stratification. The adverse-risk patients had a disappointing CR rate of 43% to 45% under age of 60.4,5 Venetoclax (VEN), in combination with low-dose cytarabine or hypomethylating agents, has shown safety and efficacy in older patients with newly diagnosed AML ineligible for IC.6-8 The VIALE-A trial demonstrated that the combination of VEN and azacytidine led to a significant improvement in composite complete remission (CRc) rate and overall survival (OS) in older and unfit patients across all genomic risk groups, even in patients with adverse cytogenetic risk and high-risk molecular mutations.9 Therefore, an urgent need exists to study the efficacy and safety of VEN-based regimen in younger adults with newly diagnosed ELN adverse-risk AML. Both real-world data analysis and a clinical trial demonstrated that the time from diagnosis to treatment did not influence OS in AML.10,11 Furthermore, the results of the Beat AML Master trial indicate that it was a feasible approach to incorporate genomic data into treatment decisions within 7 days of diagnosis. Consequently, the aim of this trial was to evaluate the efficacy and safety of VEN plus decitabine (DEC) as induction therapy in younger adults with ELN 2017 adverse-risk AML who were screened by next-generation sequencing, multiplex reverse transcription polymerase chain reaction, and fluorescence in situ hybridization within 72 hours.
This multicenter, single-arm, phase 2 study (NCT04752527) was conducted in treatment-naive younger adults with newly diagnosed de novo ELN 2017 adverse-risk AML (supplemental Figure 1). Patients were enrolled between February 2021 and July 2022 at 2 clinical sites in China (The First Affiliated Hospital of Soochow University and Suzhou Hongci Hospital). The data cutoff for this initial analysis was 31 December 2022. In cycle 1, patients received DEC 20 mg/m2 intravenously on days 1 to 5 and VEN orally 100 mg on day 1, 200 mg on day 2, and 400 mg on days 3 to 28. For patients with a high FLT3-internal tandem duplication (FLT3-ITD) allelic ratio (AR), sorafenib was optionally administered orally at a dose of 400 mg twice daily on days 3 to 28. Nonresponders or patients who achieved partial remission received 1 additional cycle of VEN (400 mg) on days 1 to 28 and DEC (20 mg/m2) on days 1 to 5. Eligible patients were offered allogeneic hematopoietic stem cell transplantation (allo-HSCT) after 1 or 2 cycles of consolidation with a high dose of cytarabine (2 g/m2, every 12 h) on days 1 to 3. Bone marrow aspirate was evaluated on either day 21 or day 28 in cycle 1 and again 1 to 2 weeks after count recovery if the day 21 or day 28 marrow was aplastic. All patients provided written informed consent in accordance with the Declaration of Helsinki. The primary outcome was CRc (defined as CR plus CR with incomplete blood count recovery [CRi] and morphologic leukemia free state [MLFS]). Secondary outcomes were OS, event-free survival (EFS), duration of remission (DOR) and safety.
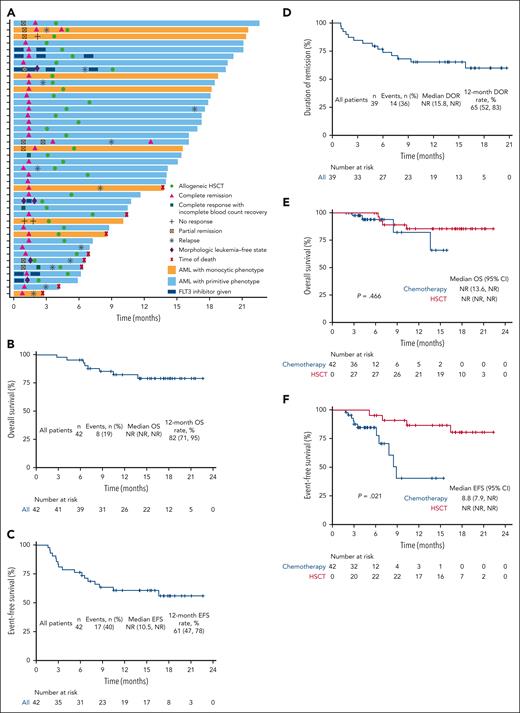
Between 12 February 2021 and 31 July 2022, 286 patients aged between 18 and 59 with newly diagnosed AML underwent screening; 78 patients were classified as ELN adverse-risk category and 42 patients were enrolled, comprising 26 male and 16 female patients (supplemental Table 2). Median age was 39 years (range 19-58). Detailed mutational landscapes and dynamic responses assessment of enrolled patients are presented in supplemental Figure 2 and Figure 1A. Ninety-three percent (39/42) of patients achieved CRc after 2 courses of therapy, and the CRc was achieved in 79% (33/42) of the patients before the initiation of cycle 2 (Table 1). Seven percent (3/42) patients did not achieve CRc after 2 cycles of induction therapy (supplemental Table 4). Among the patients with CRc, 31 (79%) patients had measurable residual disease (MRD) of <10−3 by multiparameter flow cytometry; the MRD negativity was observed in 76% of the patients before the beginning of cycle 2. At a median follow-up of 15.3 months (range 2.8-22.6), the median DOR, EFS, and OS were not reached, and the estimated 12-month OS, EFS, and DOR were 82%, 61%, and 65%, respectively (Figure 1B-D). At the data cutoff, 29% (12/42) of patients had morphologic relapse. Thirty-six (86%) patients received allo-HSCT; among them, 29 patients were offered allo-HSCT after first CR, 5 received HSCT after morphologic relapse, and 2 with salvaged HSCT. The median time from enrollment to allo-HSCT was 4.5 months (range 1.9-9.1). Using Mantel-Byar test, allo-HSCT was identified as a positive predictor for EFS (P = .021, Figure 1F).
Clinical and response characteristics of all patients enrolled in the phase 2 study. (A) Swimmer plot of dynamic response assessment. (B) OS of all patients. (C) DOR for patients who had CCr (CR + CRi + MLFS). (D) EFS of all patients. (E) Simon-Makuch plots of the impact of HSCT on OS. (F) Simon-Makuch plots of the impact of HSCT on EFS. NR, not reached.
Clinical and response characteristics of all patients enrolled in the phase 2 study. (A) Swimmer plot of dynamic response assessment. (B) OS of all patients. (C) DOR for patients who had CCr (CR + CRi + MLFS). (D) EFS of all patients. (E) Simon-Makuch plots of the impact of HSCT on OS. (F) Simon-Makuch plots of the impact of HSCT on EFS. NR, not reached.
Response assessment
| . | Overall (n = 42) . | AML with primitive phenotype (n = 33) . | AML with monocytic phenotype (n = 9) . |
|---|---|---|---|
| CRc rate | 39/42 (93% [81-99]) | 32/33 (97% [80-100]) | 7/9 (78% [40-97]) |
| CR | 34/42 (81% [66-91]) | 27/33 (82% [65-93]) | 7/9 (78% [40-97]) |
| CRi | 1/42 (2% [0-13]) | 1/33 (3% [0-16]) | 0 |
| MLFS | 4/42 (10% [3-23]) | 4/33 (12% [3-28]) | 0 |
| Partial remission | 1/42 (2% [0-13]) | 1/33 (3% [0-16]) | 0 |
| No response | 2/42 (5% [1-16]) | 0 | 2/9 (22% [3-60]) |
| MRD negativity after induction | 31/39 (79% [64-91]) | 25/32 (78% [60-91]) | 6/7 (86% [42-100]) |
| MRD negativity from all enrolled patients | 31/42 (74% [58-86]) | 25/33 (76% [58-89]) | 6/9 (67% [30-93]) |
| CRc after cycle 1 | 33/42 (79% [63-90]) | 28/33 (85% [68-95]) | 5/9 (56% [21-86]) |
| CR | 29/42 (69% [53-82]) | 24/33 (73% [54-87]) | 5/9 (56% [21-86]) |
| CRi | 2/42 (5% [1-16]) | 2/33 (6% [1-20]) | 0 |
| MLFS | 2/42 (5% [1-16]) | 2/33 (6% [1-20]) | 0 |
| Partial remission after cycle 1 | 8/42 (19% [9-34]) | 5/33 (15% [5-32]) | 3/9 (33% [7-70]) |
| No response after cycle 1 | 1/42 (2% [0-13]) | 0 | 1/9 (11% [0-48]) |
| MRD negativity after cycle 1 | 25/33 (76% [58-89]) | 21/28 (75% [55-89]) | 4/5 (80% [28-99]) |
| MRD negativity from all enrolled patients after cycle 1 | 25/42 (60% [43-74]) | 21/33 (64% [45-80]) | 4/9 (44% [14-79]) |
| Mortality at 30 days | 0 | 0 | 0 |
| Patients who received antifungal therapy | 24/42 (57% [41-72]) | 21/33 (64% [45-80]) | 3/9 (33% [7-70]) |
| High-dose Ara-C given after CRc | 30/42 (71% [55-84]) | 24/33 (73% [54-87]) | 6/9 (67% [30-93]) |
| 1-2 cycles | 26/42 (62% [46-76]) | 22/33 (67% [48-82]) | 4/9 (44% [14-79]) |
| 3 or more cycles | 4/42 (10% [3-23]) | 2/33 (6% [1-20]) | 2/9 (22% [3-60]) |
| Patients who received allogeneic HSCT | 36/42 (86% [71-95]) | 29/33 (88% [72-97]) | 7/9 (78% [40-97]) |
| Time to blood cell count recovery∗ after induction, days | 34.0 (26.0-42.0) | 34.0 (26.0-42.0) | 30 (28.0-41.5) |
| Time to absolute neutrophil count recovery to ≥1000 cells per μL, days | 31.0 (26.0-41.5) | 32.5 (26.0-41.3) | 30 (28.0-41.5) |
| Time to platelet count recovery to 50 000 platelets per μL, days | 20.0 (17.0-28.0) | 20.0 (17-33.25) | 19.0 (9.5-22.0) |
| . | Overall (n = 42) . | AML with primitive phenotype (n = 33) . | AML with monocytic phenotype (n = 9) . |
|---|---|---|---|
| CRc rate | 39/42 (93% [81-99]) | 32/33 (97% [80-100]) | 7/9 (78% [40-97]) |
| CR | 34/42 (81% [66-91]) | 27/33 (82% [65-93]) | 7/9 (78% [40-97]) |
| CRi | 1/42 (2% [0-13]) | 1/33 (3% [0-16]) | 0 |
| MLFS | 4/42 (10% [3-23]) | 4/33 (12% [3-28]) | 0 |
| Partial remission | 1/42 (2% [0-13]) | 1/33 (3% [0-16]) | 0 |
| No response | 2/42 (5% [1-16]) | 0 | 2/9 (22% [3-60]) |
| MRD negativity after induction | 31/39 (79% [64-91]) | 25/32 (78% [60-91]) | 6/7 (86% [42-100]) |
| MRD negativity from all enrolled patients | 31/42 (74% [58-86]) | 25/33 (76% [58-89]) | 6/9 (67% [30-93]) |
| CRc after cycle 1 | 33/42 (79% [63-90]) | 28/33 (85% [68-95]) | 5/9 (56% [21-86]) |
| CR | 29/42 (69% [53-82]) | 24/33 (73% [54-87]) | 5/9 (56% [21-86]) |
| CRi | 2/42 (5% [1-16]) | 2/33 (6% [1-20]) | 0 |
| MLFS | 2/42 (5% [1-16]) | 2/33 (6% [1-20]) | 0 |
| Partial remission after cycle 1 | 8/42 (19% [9-34]) | 5/33 (15% [5-32]) | 3/9 (33% [7-70]) |
| No response after cycle 1 | 1/42 (2% [0-13]) | 0 | 1/9 (11% [0-48]) |
| MRD negativity after cycle 1 | 25/33 (76% [58-89]) | 21/28 (75% [55-89]) | 4/5 (80% [28-99]) |
| MRD negativity from all enrolled patients after cycle 1 | 25/42 (60% [43-74]) | 21/33 (64% [45-80]) | 4/9 (44% [14-79]) |
| Mortality at 30 days | 0 | 0 | 0 |
| Patients who received antifungal therapy | 24/42 (57% [41-72]) | 21/33 (64% [45-80]) | 3/9 (33% [7-70]) |
| High-dose Ara-C given after CRc | 30/42 (71% [55-84]) | 24/33 (73% [54-87]) | 6/9 (67% [30-93]) |
| 1-2 cycles | 26/42 (62% [46-76]) | 22/33 (67% [48-82]) | 4/9 (44% [14-79]) |
| 3 or more cycles | 4/42 (10% [3-23]) | 2/33 (6% [1-20]) | 2/9 (22% [3-60]) |
| Patients who received allogeneic HSCT | 36/42 (86% [71-95]) | 29/33 (88% [72-97]) | 7/9 (78% [40-97]) |
| Time to blood cell count recovery∗ after induction, days | 34.0 (26.0-42.0) | 34.0 (26.0-42.0) | 30 (28.0-41.5) |
| Time to absolute neutrophil count recovery to ≥1000 cells per μL, days | 31.0 (26.0-41.5) | 32.5 (26.0-41.3) | 30 (28.0-41.5) |
| Time to platelet count recovery to 50 000 platelets per μL, days | 20.0 (17.0-28.0) | 20.0 (17-33.25) | 19.0 (9.5-22.0) |
Data are presented as n/N (% [95% confidence interval]) or median (interquartile range).
Blood cell count recovery was defined as an absolute neutrophil count ≥ 1000 cells/μL and a platelet count ≥ 50 000 platelets/μL.
Efficacy outcomes by subgroups are shown in supplemental Figures 3 and 4. Excitingly, we observed high CRc rates in some subgroups after cycle 1, including CRc rates of 88% in patients with complex karyotype and 83% in patients with RUNX1 mutations, especially in those with a single RUNX1 mutation (100%). Single RUNX1 mutations might be associated with better outcomes in the modern era with VEN-based regimens, similar to those described by Cherry et al.12 and Venugopal et al.13 In addition, the response rates in TP53 mutations, spliceosome mutations, and FLT3-ITDAR≥0.5 were also significantly improved in this study. Five patients with FLT3-ITDAR≥0.5 who received triple therapy had a CRc rate of 100% (CR, 60%; CRi, 0%; and MLFS, 40%), which was similar to that reported by Stone et al in the RATIFY study.14 However, patients with monocytic phenotype AML had a lower CRc rate in course 1 than those with primitive phenotype (56% vs 85%). Two patients with monocytic phenotype showed primary resistance to VEN + DEC, in accordance with the observations by Cherry et al12 and Pei et al.15 However, due to the small numbers of this population, this status was not found to be a statistically predictor for CRc both by univariate (P = .070) and multivariate logistics regression models (P = .053, supplemental Table 5). The CRc rate in patients with KMT2A rearrangement (KMT2Ar) was 67%, which was much lower than those reported by DiNardo et al and Wang et al.16,17 Fifty-six percent of patients with KMT2Ar had early relapse, and the median DOR was 1.8 months. KMT2Ar status was a statistically significant predictor for short DOR in both univariate (P = .002) and multivariate analyses (P = .004, supplemental Table 6). It was also a significant predictor of unfavorable outcome in both univariate (P = .003) and multivariate analyses (P = .001, supplemental Table 7). Together, these results suggested that patients with KMT2Ar should proceed to allo-HSCT after first remission to decrease the chances of early relapse.
Overall, 42 patients were included in the safety analysis (supplemental Table 8). With cytoreduction before starting therapy to reduce the white blood cell count below 25 × 109/L, tumor lysis syndrome was not observed. The most common hematologic adverse events of grade 3 or worse were thrombocytopenia in 30 patients (91%), neutropenia in 29 patients (100%), and anemia in 29 patients (91%). Notable serious adverse events included pneumonia, febrile neutropenia, and sepsis, which were observed in 14%, 26%, and 2% of patients, respectively. This regimen presented a lower toxicity profile than those reported in other clinical trials with intensive regimen.18,19 Moreover, the median time to recovery of 50 × 109/L or more platelets was 20 days (interquartile range 17.0-28.0) after induction, which was shorter than that described in the CLIA trial and similar to that described in the DAV trial.17,20 Assessment of patient mortality revealed that the 30-day and 60-day mortality rates were both 0%.
In summary, we report the first study of VEN plus DEC in younger adults with newly diagnosed ELN adverse-risk AML, and this regimen was effective and well tolerated. The improved CRc and outcome in patients with FLT3-ITDAR≥0.5 highlighted the potential benefits of FLT3 inhibitors in combination with VEN-based regimens, and further validation is needed. Especially, FLT3 status alone no longer constitutes adverse risk under ELN 2022. These effect sizes may not be reliable given the small number of total subjects and subgroups. A phase 3 randomized study will soon be conducted to compare this regimen to IC in patients with newly diagnosed ELN adverse-risk AML.
Acknowledgments
The authors thank the patients, their families, and their caregivers; coinvestigators; collaborators; and members of the study team involved in this trial.
This work was supported by grants from the National Key Research and Development Program of China (2019YFC0840604), grants from the Key Research and Development Program of Jiangsu Province (BE2019798), and grants from the National Natural Science Foundation of China (82020108003 and 82170158).
Authorship
Contribution: D.W., S.C., X.Y., and J.X. conceived and designed the study; J.X., X.Y., S.-L.X., H.S., J.C., L.Y., J.P., M.Z., D.L., X. Hu, Q.W., J.Z., H.D., Y.C., X. He, X.T., A.S., Y.W., J.F., H.Q., S.C., and D.W. provided study materials or patients; J.X., X.Y., X.B., S.-L.X., and H.S. collected and assembled data; J.X., X.Y., X.B., and S.C. performed data analysis and interpretation; and all authors contributed to revision of the manuscript, gave final approval of manuscript, are accountable for all aspects of the work, had full access to all the data in the study, and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Depei Wu, National Clinical Research Center for Hematologic Diseases, The First Affiliated Hospital of Soochow University, Soochow University, Shizi Street 188, Suzhou, 215006, China; e-mail: drwudepei@163.com; Suning Chen, National Clinical Research Center for Hematologic Diseases, The First Affiliated Hospital of Soochow University, Soochow University, Shizi Street 188, Suzhou, 215006, China; e-mail: chensuning@suda.edu.cn; and Xiaofei Yang, National Clinical Research Center for Hematologic Diseases, The First Affiliated Hospital of Soochow University, Soochow University, Shizi Street 188, Suzhou, 215006, China; e-mail: yangxiaofei@suda.edu.cn.
References
Author notes
∗J.X., X.B., S.-L.X, and H.S. contributed equally to this work.
These data were presented as an oral abstract in part at the 63rd Annual Meeting of the American Society of Hematology, 11 to 14 December 2021 (Atlanta, GA), and the 64th Annual Meeting of the American Society of Hematology, 10 to 13 December 2022 (New Orleans, LA).
ClinicalTrials.gov identifier: NCT04752527.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal