Abstract
Platelets are key vascular effectors in hemostasis, with activation signals leading to fast recruitment, aggregation, and clot formation. The canonical process of hemostasis is well-characterized and shares many similarities with pathological thrombus formation. However, platelets are also crucially involved in the maintenance of vascular integrity under both steady-state and inflammatory conditions by ensuring blood vessel homeostasis and preventing microbleeds. In these settings, platelets use distinct receptors, signaling pathways, and ensuing effector functions to carry out their deeds. Instead of simply forming clots, they mainly act as individual sentinels that swiftly adapt their behavior to the local microenvironment. In this review, we summarize previously recognized and more recent studies that have elucidated how anucleate, small platelets manage to maintain vascular integrity when faced with challenges of infection, sterile inflammation, and even malignancy. We dissect how platelets are recruited to the vascular wall, how they identify sites of injury, and how they prevent hemorrhage as single cells. Furthermore, we discuss mechanisms and consequences of platelets’ interaction with leukocytes and endothelial cells, the relevance of adhesion as well as signaling receptors, in particular immunoreceptor tyrosine–based activation motif receptors, and cross talk with the coagulation system. Finally, we outline how recent insights into inflammatory hemostasis and vascular integrity may aid in the development of novel therapeutic strategies to prevent hemorrhagic events and vascular dysfunction in patients who are critically ill.
Introduction
Platelets were long regarded solely as cellular components of primary hemostasis that form clots to prevent blood loss but which also cause ischemic injury in the setting of intravascular thrombosis. In combination with their unique receptor repertoire, short half life and limited ability for translation has propelled platelets to the forefront of pharmacological intervention in (arterial) thrombotic disease. However, research of recent decades has revealed unexpected new aspects of platelet biology, eg, these small, anucleate cell fragments are unique in their rapid recruitment dynamics, making them the first cells to appear not only at sites of injury but also inflammation.1 Moreover, they can exert forces in the range of 30 to 50 nN and use their unique signaling cascades to constantly scan and respond to their microenvironment, combined with heterogenous cargo, including cytokines and growth hormones.2
This diverse skill set enables platelets to act as key players in the setting of sterile or pathogen-induced inflammation.3-6 In this study, platelets can serve as immune sentinels that autonomously fight invading microbes through effector functions, such as migration-mediated pathogen bundling, secretion of microbicidal agents, and orchestration of leukocyte recruitment.7 Platelets are also key components that license neutrophils to form neutrophil extracellular traps, which in turn promote immunothrombosis, which is classically defined as a phenomenon characterized by the cooperation and reciprocal activation of platelets, coagulation factors, innate immune cells, and endothelial cells aiming to not only prevent pathogen dissemination but also contributes to occlusive clot formation in arterial thrombosis.3,8,9 Beyond innate immunity, platelets even shape adaptive immune responses to viruses and fungi through antigen presentation and influencing lymphocyte polarization.10-23
Focusing on the role of platelets in inflammation has brought to light 1 novel, key role of platelets, which is the focus of this review, the maintenance of vessel integrity without the formation of a thrombus, which is key in acute challenges to the vasculature, such as inflammation, but that surprisingly extends to homeostatic conditions as well.
In this review, we summarize the complex pathophysiology that underlies inflammation-associated hemorrhage and describe how single platelets manage to counteract blood loss. We focus on the tissue- and model-dependent differences in inflammatory hemostasis and highlight the variety of (i) platelet receptors and signaling hubs as well as (ii) specific platelet functions that have been shown to mediate hemostatic effects in inflammation. Moreover, we summarize studies that have uncovered platelet-mediated vascular integrity in the absence of overt injury as well as inflammation models, showing the contribution of both novel and well-known platelet functions, such as platelet migration and procoagulant activation (PA).
Canonical platelet responses in classical thrombosis and hemostasis
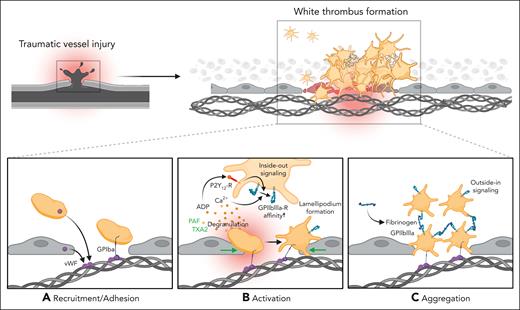
Thrombus formation after traumatic vessel injury is a well-characterized process (Figure 1). After vascular damage, exposed subendothelial extracellular matrix proteins, such as collagen and fibronectin, are exposed. Binding of plasma-, endothelial cell- and platelet-derived von Willebrand factor (vWF) to exposed collagen facilitates recruitment of incoming platelets through the vWF-binding glycoprotein GPIbα that mediates initial tethering.24-26 After this binding, a complex web of signaling cascades within the individual platelet is set into motion, which promote inside-out signaling pathways, integrin GPIIBIIIA activation and platelet degranulation.27 Subsequently, platelet recruitment, activation, and aggregation are boosted through platelet-platelet binding through soluble and released fibrinogen-GPIIBIIIA interaction and activators, adenosine 5′-diphosphate and thromboxane, which act both in an auto and a paracrine manner and crucially contribute to organizing and stabilizing the growing thrombus.26,28 Notably, detrimental thrombus formation after atherosclerotic plaque rupture relies on the same platelet receptors and downstream signaling cascades.25
Mechanisms of classical hemostasis after traumatic vessel injury. (A) Traumatic injury leads to exposure of extracellular matrix (ECM) proteins, such as collagen and binding of von Willebrand factor (vWF) derived from endothelial cells and platelets. Adhesion of incoming platelets to the injured vessel wall is mediated by the vWF-binding glycoprotein GPIbα. (B) Activation of recruited platelets and distinct shape change through auto- and paracrine secretion of prothrombotic factors, such as adenosine 5′-diphosphate, leading to an increased affinity of the αIIbβ3/GPIIBIIIA receptor. (C) Binding of fibrinogen activates integrin inside-out signaling pathways, thus promoting aggregation of neighboring platelets and resulting in a stable thrombus formation.
Mechanisms of classical hemostasis after traumatic vessel injury. (A) Traumatic injury leads to exposure of extracellular matrix (ECM) proteins, such as collagen and binding of von Willebrand factor (vWF) derived from endothelial cells and platelets. Adhesion of incoming platelets to the injured vessel wall is mediated by the vWF-binding glycoprotein GPIbα. (B) Activation of recruited platelets and distinct shape change through auto- and paracrine secretion of prothrombotic factors, such as adenosine 5′-diphosphate, leading to an increased affinity of the αIIbβ3/GPIIBIIIA receptor. (C) Binding of fibrinogen activates integrin inside-out signaling pathways, thus promoting aggregation of neighboring platelets and resulting in a stable thrombus formation.
This process, termed primary hemostasis, is accompanied by and supports the activation of plasmatic coagulation pathways, namely the tissue factor/factor VII (FVII)–mediated extrinsic pathway as well as the FXII–induced intrinsic pathway (reviewed elsewhere).26,29 Together, concomitant activation of both cellular and plasmatic coagulation effectively seals traumatic vessel injury, prevents potentially lethal bleeding, and serves as a basis for vascular recovery (Figure 1). It is worth noting that moderately decreasing platelet counts per se do not necessarily exacerbate trauma-induced bleeding, suggesting system robustness even when platelet numbers are low. In mice, only severe thrombocytopenia (<10% of normal platelet counts) was consistently associated with prolonged bleeding times.30
Platelet-mediated hemostasis without clot formation: a complex and heterogenous process
As the central role of platelets in inflammation and infection became more and more apparent, landmark papers by Goerge et al and Ho-Tinh-Noe et al demonstrated that platelet depletion in different murine models of inflammation led to significant bleeding (refer to Table 1 for an overview of the most commonly used models).31,32 Interestingly, intravital imaging revealed that platelets do not form clots in the inflamed microvasculature but rather are recruited and act as individual cells, underlining that the hemostatic process is distinct from classical thrombosis and hemostasis. In contrast to the well-defined processes of hemostasis after vascular trauma, inflammation-associated hemorrhage, also coined inflammatory bleeding, is a simplified summary of a phenomenon that occurs across a variety of disease settings, including sterile inflammation, microbial infection, and malignant tumors.33-35
Murine models of inflammatory bleeding
| Murine models used . | Description . | Role of platelets in preventing hemorrhage . | Role of platelets in leukocyte recruitment . | Clinical relevance . | Reference . |
|---|---|---|---|---|---|
| Cerebral IRI-tMCAO | Transient occlusion of the middle cerebral artery is realized by introducing a filament into the artery | Conflicting data, with thrombocytopenia being associated with microbleeds in one study, infarct protection in another | Unclear | Secondary hemorrhage is a highly relevant and dreaded complication of ischemic stroke | 30,36 |
| Acute lung injury (acid-induced) | Alveolar instillation of hydrochloric acid, causing sterile inflammation and injury | No data available | Leukocyte recruitment depends on presence of platelets | Pulmonary hemorrhage in the context of acute lung injury is a relevant and challenging clinical scenario | 37 |
| Acute lung injury (LPS-induced) | Alveolar LPS instillation, mimicking infectious causes of acute lung injury | Severe alveolar bleeding observed in thrombocytopenia | Leukocyte recruitment not affected by thrombocytopenia | See above | 36,38,39 |
| Dermal rpA | Model of immune complex–mediated acute inflammatory tissue injury by antigen/antibody coinjection | Severe cutaneous bleeding observed in thrombocytopenia | Platelets orchestrate leukocyte recruitment in this model | Immune complex–mediated bleeding of minor clinical relevance | 36,40 |
| Pulmonary rpA | See above; intracheal instillment | No effect reported | No effect reported | Immune complex–mediated bleeding of minor clinical relevance | 41 |
| Peritoneal rpA | See above; intraperitoneal instillment | Severe abdominal bleeding in thrombocytopenia | Reduced leukocyte recruitment in thrombocytopenia | Immune complex–mediated bleeding of minor clinical relevance | 42 |
| Tumor microvasculature | Induction of local malignoma and subsequent assessment of tumor microvasculature | Thrombocytopenia associated with hemorrhage, might be associated with higher efficiency of chemotherapy | No effect reported | Malignancy associated bleeding is a relevant clinical problem particularly in advanced tumor stages | 43,44 |
| Peritonitis (LPS-induced) | Injection of LPS into the peritoneal cavity | Inconclusive results, thrombocytopenia might result in microbleeding | Platelet depletion severely reduces leukocyte recruitment | Infection-associated peritoneal hemorrhage of minor relevance | 39,45 |
| UV-B induced dermal injury | UV-B exposure of the skin | Thrombocytopenia causes bleeding | No effect reported | Of minor clinical relevance | 46 |
| Cremasteric inflammation (LPS- or cytokine-induced) | LPS/cytokines are injected intrascrotally and the cremaster muscle is exposed for imaging/whole mount microscopy | Platelets are required to prevent microbleeds | Platelets are crucial for leukocyte recruitment in this model | Diffuse muscle bleeding is rather rare and of minor clinical importance | 38,47 |
| Inflammatory colitis | Oral administration of DSS | Platelets are required to prevent microbleeds | No data available | Major relevance of hemodynamically relevant intestinal bleeding in patients with inflammatory bowel diseases | 48 |
| Murine models used . | Description . | Role of platelets in preventing hemorrhage . | Role of platelets in leukocyte recruitment . | Clinical relevance . | Reference . |
|---|---|---|---|---|---|
| Cerebral IRI-tMCAO | Transient occlusion of the middle cerebral artery is realized by introducing a filament into the artery | Conflicting data, with thrombocytopenia being associated with microbleeds in one study, infarct protection in another | Unclear | Secondary hemorrhage is a highly relevant and dreaded complication of ischemic stroke | 30,36 |
| Acute lung injury (acid-induced) | Alveolar instillation of hydrochloric acid, causing sterile inflammation and injury | No data available | Leukocyte recruitment depends on presence of platelets | Pulmonary hemorrhage in the context of acute lung injury is a relevant and challenging clinical scenario | 37 |
| Acute lung injury (LPS-induced) | Alveolar LPS instillation, mimicking infectious causes of acute lung injury | Severe alveolar bleeding observed in thrombocytopenia | Leukocyte recruitment not affected by thrombocytopenia | See above | 36,38,39 |
| Dermal rpA | Model of immune complex–mediated acute inflammatory tissue injury by antigen/antibody coinjection | Severe cutaneous bleeding observed in thrombocytopenia | Platelets orchestrate leukocyte recruitment in this model | Immune complex–mediated bleeding of minor clinical relevance | 36,40 |
| Pulmonary rpA | See above; intracheal instillment | No effect reported | No effect reported | Immune complex–mediated bleeding of minor clinical relevance | 41 |
| Peritoneal rpA | See above; intraperitoneal instillment | Severe abdominal bleeding in thrombocytopenia | Reduced leukocyte recruitment in thrombocytopenia | Immune complex–mediated bleeding of minor clinical relevance | 42 |
| Tumor microvasculature | Induction of local malignoma and subsequent assessment of tumor microvasculature | Thrombocytopenia associated with hemorrhage, might be associated with higher efficiency of chemotherapy | No effect reported | Malignancy associated bleeding is a relevant clinical problem particularly in advanced tumor stages | 43,44 |
| Peritonitis (LPS-induced) | Injection of LPS into the peritoneal cavity | Inconclusive results, thrombocytopenia might result in microbleeding | Platelet depletion severely reduces leukocyte recruitment | Infection-associated peritoneal hemorrhage of minor relevance | 39,45 |
| UV-B induced dermal injury | UV-B exposure of the skin | Thrombocytopenia causes bleeding | No effect reported | Of minor clinical relevance | 46 |
| Cremasteric inflammation (LPS- or cytokine-induced) | LPS/cytokines are injected intrascrotally and the cremaster muscle is exposed for imaging/whole mount microscopy | Platelets are required to prevent microbleeds | Platelets are crucial for leukocyte recruitment in this model | Diffuse muscle bleeding is rather rare and of minor clinical importance | 38,47 |
| Inflammatory colitis | Oral administration of DSS | Platelets are required to prevent microbleeds | No data available | Major relevance of hemodynamically relevant intestinal bleeding in patients with inflammatory bowel diseases | 48 |
DSS, dextran sodium sulfate; IRI, ischemia-reperfusion injury; LPS, lipopolysaccharide; rpA, reverse-passive Arthus reaction.
Whether or not vascular integrity is maintained and whether bleeding occurs heavily depends on tissue- and niche-specific factors. In most settings studied, leukocyte transmigration is responsible for disrupting endothelial patency. Importantly, this complicates assessment of the role of platelets in inflammatory hemostasis, because these are also required for preceding immune cell recruitment in some models (refer to Table 1).49 In contrast to arterial thrombosis or hemostatic plug formation after traumatic injury, which require canonical platelet responses, inflammatory hemostasis in 1 organ may require platelet functions that may be dispensable in preserving vascular integrity in another.33,50 Importantly, the 1 unifying factor that has been shown to crucially exacerbate hemorrhage in most animal models is thrombocytopenia, which also associated with inflammatory bleeding in the brain and lungs of patients with severe inflammation (Figure 2).4,31,32,51 However, it is important to note that although thrombocytopenia is the most important aggravating factor of inflammation-associated hemorrhage in most settings, some organs have been shown to be unsusceptible to inflammatory bleeding even in severe thrombocytopenia, including models, such as thioglycolate- or immune complex–induced peritonitis, arthritis, and endotoxemia.36,42,43,52 In the following paragraphs, we summarize insights into mechanisms of initial recruitment, repositioning, sensing, and prevention of bleeding by platelets in the context of inflammatory hemostasis.
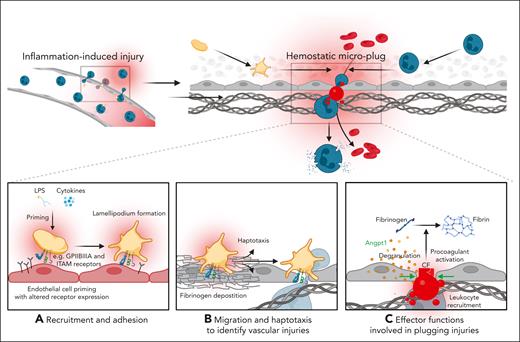
A mechanistic model of key platelet functions in inflammatory hemostasis. (A) Proinflammatory mediators, such as exogenous LPS or endogenous cytokines prime platelets to express a distinct set of receptors, facilitating their adhesion to the inflamed vessel wall and migration is initiated by lamellipodium formation. (B) Through migration and haptotaxis-mediated sensing of density gradients of deposited fibrinogen, platelets detect neutrophil transmigrations sites, which cause vascular breaches and subsequent leakage of red blood cells. (C) Exposed ECM proteins trigger further activation, such as degranulation and PA. Single platelets physically plug injury sites and influence leukocyte recruitment through direct receptor interaction and endothelial-platelet cross talk. Secretion of soluble mediators such as angiopoietin-1 (Angpt1) stabilizes endothelial cell junctions, preventing leukocyte transmigration. Plasmatic coagulation factors such as fibrinogen and thrombin are recruited to the surface of PS–positive procoagulant platelets, which foster local fibrin deposition and act as a single hemostatic plug that further seal endothelial microlesions. We note that this mechanistic model has been shown in LPS-induced acute lung injury and inflammation of both the peritoneum and the cremasteric microcirculation; its relevance in other models of inflammatory bleeding is yet to be examined.
A mechanistic model of key platelet functions in inflammatory hemostasis. (A) Proinflammatory mediators, such as exogenous LPS or endogenous cytokines prime platelets to express a distinct set of receptors, facilitating their adhesion to the inflamed vessel wall and migration is initiated by lamellipodium formation. (B) Through migration and haptotaxis-mediated sensing of density gradients of deposited fibrinogen, platelets detect neutrophil transmigrations sites, which cause vascular breaches and subsequent leakage of red blood cells. (C) Exposed ECM proteins trigger further activation, such as degranulation and PA. Single platelets physically plug injury sites and influence leukocyte recruitment through direct receptor interaction and endothelial-platelet cross talk. Secretion of soluble mediators such as angiopoietin-1 (Angpt1) stabilizes endothelial cell junctions, preventing leukocyte transmigration. Plasmatic coagulation factors such as fibrinogen and thrombin are recruited to the surface of PS–positive procoagulant platelets, which foster local fibrin deposition and act as a single hemostatic plug that further seal endothelial microlesions. We note that this mechanistic model has been shown in LPS-induced acute lung injury and inflammation of both the peritoneum and the cremasteric microcirculation; its relevance in other models of inflammatory bleeding is yet to be examined.
Receptors involved in platelet recruitment in inflammatory hemostasis
The recruitment of platelets under inflammatory conditions requires distinct receptors and downstream signaling cascades. Importantly, the receptors used by circulating and recruited platelets heavily rely on (1) the inflammatory stimulus and (2) the organ-specific vascular bed.
Classical hemostasis crucially depends on binding of platelet GPIb to vWF as well as platelet-platelet aggregate formation through fibrin(ogen)–mediated GPIIBIIIA interactions, and blockade or depletion of either component is known to aggravate bleeding after traumatic vessel injury. However, in inflammatory bleeding, the above receptors are dispensable in some settings.
The impact of GPIIBIIIA on inflammatory hemostasis is dichotomous and ranges from no effect to crucially aggravating hemorrhage. Whole-body knockout of α IIb integrins exacerbated pulmonary bleeding upon lipopolysaccharide (LPS) stimulation, whereas irreversible inhibition of GPIIBIIIA using the clinically approved antagonist eptifibatide (integrillin) in the same model showed only modest increases in pulmonary hemorrhage.50,53 Enhanced bleeding was also observed in LPS-induced inflammation of the cremasteric microcirculation when mice were treated with repetitive injections of the reversible GPIIBIIIA antagonist tirofiban.53 In patients with stroke, antibody-mediated GPIIBIIIIA neutralization using abciximab did not improve neurological outcomes but significantly increased the rate of symptomatic or lethal intracranial hemorrhage.54 Although GPIIBIIIA protected from hemorrhage in models of cerebral ischemic-perfusion injury and a clinically relevant model of lymphocytic choriomeningitis virus (LCMV) infection, GPIIBIIIA did not contribute to inflammatory hemostasis in models of dermal reverse-passive Arthus reaction (rpA) or ovalbumin-mediated immunization.45,50,55-57
Similarly, GPIb and its main binding partner vWF have been shown to differentially affect inflammatory hemostasis. Although being important regulators of thromboinflammation in ischemic stroke, cerebral hemorrhage in the same ischemia-reperfusion model as described above was not dependent on either GPIb or vWF.57,58 In acute lung injury, whole-body vWF knockout mice showed increased hemorrhage, and antibody-mediated vWF neutralization aggravated bleeding in dermal rpA.50 In the tumor vasculature, in sterile (glomerulonephritis) or pathogen-driven inflammation (LCMV infection), GPIb was dispensable for maintaining vascular integrity.31,35,45,56,59 These partially diverging findings may be explainable through a recent study describing platelet PA through S100A8/9-mediated GPIb signaling in severe infection, which adds further complexity to inflammatory hemostasis and may serve as an interesting therapeutic target for further investigation.38
The 2 (hemi)immunoreceptor tyrosine–based activation motif (ITAM) receptors GPVI and CLEC-2 are highly expressed in both human and murine platelets.16,60,61 The fact that both have been shown to be dispensable for classical hemostasis after traumatic vessel injury, whereas being targetable in thrombus formation has sparked interest in their noncanonical functions. Consequently, their role in inflammation and associated bleeding models have been investigated thoroughly.16,60,62-64 Boulaftali et al described a crucial role for CLEC-2, GPVI, and their downstream signal transductor Src-homology leucocyte protein 76 on maintaining vascular integrity in both the dermal rpA model as well as LPS-induced acute lung injury, with a negligible role for G–receptor coupled signaling.65 Pharmacologically inhibiting Bruton tyrosine kinase, another downstream mediator of (hemi) ITAM receptors, in transfused platelets reduced inflammatory bleeding, suggesting a negligible role for this part of (hemi) ITAM signaling in both acute lung injury and dermal rpA reaction.66 We note that these observations were made in the hIL-4Rα/GPIbα transfusion model, in which mice do not reach steady-state platelet counts despite transfusion. Although the 2 studies mentioned so far focused on assessing bleeding after a certain time point, a pivotal study by Gros et al investigated the role of GPVI in dermal rpA using live imaging of platelets and neutrophils.42 In this study, GPVI was found to be essential for modulating neutrophil infiltration and for recruiting single platelets to the sites of neutrophil extravasation. Interestingly, immune complex–mediated peritonitis showed that neutrophil myeloperoxidase and reactive oxygen species activity in the peritoneal cavity depended on platelet GPVI but failed to induce inflammatory hemorrhage even in the absence of platelets or platelet GPVI.42 GPVI also contributes to vascular hemostasis in malignancy, as discussed in subsequent section.67 Interestingly, dual inhibition of GPVI and either CLEC-2 or GPIIBIIIA has been shown to aggravate inflammatory bleeding after dermal rpA and acute lung injury, respectively.50,68 However, we also note that although Boulaftali et al provided convincing data on the role of GPVI in pulmonary hemorrhage, neither Rayes et al nor we observed substantial inflammatory bleeding after LPS- or rpA-induced acute lung injury in mice using either JAQ1-mediated GPVI depletion or GPVI−/− mice, which is in line with a negligible role of Bruton tyrosine kinase–mediated signaling.50,65,66,68 Burkard et al recently provided similar results, with no differences in alveolar hemorrhage after LPS-induced acute lung injury in either wild-type or GPVI–/– mice.69 These findings, although appearing contradictory at first sight, emphasize bleeding dependency on (1) the agent used for inducing the respective inflammation model as well as (2) duration of exposure and concentration of the respective agent.
An overview of platelet receptors, binding partners, and intracellular signaling molecules, for which the contribution to different inflammation models has been investigated, is summarized in Table 2 and visualized in Figure 3.
Relevant receptors and signaling cascades
| Receptor/ signaling hub . | Models with aggravated bleeding upon inhibition . | No bleeding observed upon inhibition . | Reference . |
|---|---|---|---|
| GPIIBIIIA | Acute lung injury (full knockout, irreversible inhibition through eptifibatide, combined with GPVI), viral infection with LCMV, cerebral IRI (tMCAO), LPS-induced peritonitis (in synergy with GPVI), LPS-induced cremaster inflammation | Dermal rpA (incl. downstream Gα13), acute lung injury (reversible, single inhibition through tirofiban), immunization (ovalbumin) | 31,32,35,45,50,53,55-57,68,70,71 |
| GPIb/vWF interaction | Acute lung injury (vWF−/−), dermal rpA (vWF-blocking antibody) | Dermal rpA, cerebral IRI (tMCAO), solid tumors, LCMV infection, glomerulonephritis | 32,49,50,56,59 |
| GPVI | Acute lung injury (more severe in synergy with GPIIBIIIA blockade), tumor bleeding, dermal rpA (in synergy with CLEC-2/podoplanin), LPS-induced peritonitis (in synergy with GPIIBIIIA) | Dermal rpA (GPVI−/−), cerebral IRI (tMCAO), pulmonary rpA | 32,42,50,65,67,68 |
| CLEC-2 | Immunization (ovalbumin), dermal rpA (only in synergy with GPVI), acute lung injury | Dermal rpA (single inhibition, podoplanin blockade) | 45,50,55,60,64,65 |
| SLP-76 | Dermal rpA, acute lung injury | — | 65 |
| PAR4 | — | Dermal rpA, acute lung injury | 52,65,72 |
| Podoplanin (as counter receptor for platelet CLEC-2) | Immunization, dermal rpA (only in synergy with GPVI deletion) | Dermal rpA (single inhibition through antibody or endothelial deletion) | 50,55,60 |
| P-selectin (CD62P) | Acid-induced acute lung injury (enhanced neutrophil recruitment, no data on bleeding) | Dermal rpA, LCMV | 32,45,56,73 |
| CalDAG-GEFI | — | Dermal rpA | 32 |
| P2Y12 | — | Dermal rpA, acute lung injury | 65 |
| P2X1 | Inflammatory colitis | Acute lung injury | 48 |
| TXA2 | — | Dermal rpA, acute lung injury | 65 |
| S1P (platelet-derived) | — | Dermal rpA, immunization (ovalbumin) | 55,74 |
| BTK | — | Dermal rpA, acute lung injury | 66 |
| Arpc2 | Acute lung injury (high-dose and low-dose LPS), CCL2-induced cremaster inflammation | — | 53 |
| c-Src | Acute lung injury | — | 75 |
| CypD | Acute lung injury | — | 68 |
| TMEM16F | Acute lung injury | — | 68 |
| Receptor/ signaling hub . | Models with aggravated bleeding upon inhibition . | No bleeding observed upon inhibition . | Reference . |
|---|---|---|---|
| GPIIBIIIA | Acute lung injury (full knockout, irreversible inhibition through eptifibatide, combined with GPVI), viral infection with LCMV, cerebral IRI (tMCAO), LPS-induced peritonitis (in synergy with GPVI), LPS-induced cremaster inflammation | Dermal rpA (incl. downstream Gα13), acute lung injury (reversible, single inhibition through tirofiban), immunization (ovalbumin) | 31,32,35,45,50,53,55-57,68,70,71 |
| GPIb/vWF interaction | Acute lung injury (vWF−/−), dermal rpA (vWF-blocking antibody) | Dermal rpA, cerebral IRI (tMCAO), solid tumors, LCMV infection, glomerulonephritis | 32,49,50,56,59 |
| GPVI | Acute lung injury (more severe in synergy with GPIIBIIIA blockade), tumor bleeding, dermal rpA (in synergy with CLEC-2/podoplanin), LPS-induced peritonitis (in synergy with GPIIBIIIA) | Dermal rpA (GPVI−/−), cerebral IRI (tMCAO), pulmonary rpA | 32,42,50,65,67,68 |
| CLEC-2 | Immunization (ovalbumin), dermal rpA (only in synergy with GPVI), acute lung injury | Dermal rpA (single inhibition, podoplanin blockade) | 45,50,55,60,64,65 |
| SLP-76 | Dermal rpA, acute lung injury | — | 65 |
| PAR4 | — | Dermal rpA, acute lung injury | 52,65,72 |
| Podoplanin (as counter receptor for platelet CLEC-2) | Immunization, dermal rpA (only in synergy with GPVI deletion) | Dermal rpA (single inhibition through antibody or endothelial deletion) | 50,55,60 |
| P-selectin (CD62P) | Acid-induced acute lung injury (enhanced neutrophil recruitment, no data on bleeding) | Dermal rpA, LCMV | 32,45,56,73 |
| CalDAG-GEFI | — | Dermal rpA | 32 |
| P2Y12 | — | Dermal rpA, acute lung injury | 65 |
| P2X1 | Inflammatory colitis | Acute lung injury | 48 |
| TXA2 | — | Dermal rpA, acute lung injury | 65 |
| S1P (platelet-derived) | — | Dermal rpA, immunization (ovalbumin) | 55,74 |
| BTK | — | Dermal rpA, acute lung injury | 66 |
| Arpc2 | Acute lung injury (high-dose and low-dose LPS), CCL2-induced cremaster inflammation | — | 53 |
| c-Src | Acute lung injury | — | 75 |
| CypD | Acute lung injury | — | 68 |
| TMEM16F | Acute lung injury | — | 68 |
Unless otherwise stated, acute lung injury was induced using LPS.
BTK, Bruton tyrosine kinase; CypD, cyclophilin D; CalDAG-GEFI, Ca2+ and DAG-regulated guanine nucleotide exchange factor I; P2X1, P2X receptor 1; P2Y12, P2Y receptor 12; PAR4, protease-activated receptor 4; S1P, sphingosine-1-phosphate; SLP-76, Src-homology leucocyte protein 76 (also known as Lymphocyte cytosolic protein 2); TMEM16F, transmembrane protein 16F; TXA2, thromboxane.
Platelet receptors and signaling cascades the protect from inflammatory hemorrhage. Increase in inflammatory bleeding by inhibition of the main receptors GPIIBIIIA, GPIb, GPVI and CLEC-2 expressed on the surface of platelets. Although single inhibition of GPIIBIIIA in models of acute lung injury, LCMV infection, cerebral ischemia–reperfusion injury (IRI) and cremaster inflammation using direct receptor antibody or whole-body knockout were sufficient, combined blockage of GPIIBIIIA and GPVI aggravated the bleeding phenotype in the models of acute lung injury and peritonitis. GPIIBIIIA outside-in signaling is targeted through the Gα13/c-Src/14-3-3ζ complex or Arpc2, relevant in actin nucleation and lamellipodium formation. GPIIBIIIA and GPVI downstream pathways increase intracellular Ca2+ concentrations in a Syk- and PLCγ2-dependent manner, resulting in PS expression on the outer platelet membrane. Inhibition of CypD, as part of the mitochondrial permeability transition pore (mPTP) formation, or transmembrane protein 16F, a calcium-dependent scramblase, decreases PA and aggravates inflammatory bleeding. Local recruitment of coagulations factors to PS-positive platelets can be directly inhibited by anti-FIIa/-FXa, interfering with inflammatory hemostasis. GPIb can be inhibited by a whole-body knockout or blocking antibody of its ligand von Willebrand factor (vWF) in acute lung injury and dermal rpA. Inhibition of CLEC-2 or its downstream effector Src-homology leucocyte protein 76 are sufficient to enhance inflammatory bleeding in acute lung injury, while in dermal rpA simultaneously blockage of CLEC-2 or its ligand podoplanin and GPVI is necessary. Ablation of both alpha and dense platelet granules is required to affect inflammatory hemostasis, eg, in cerebral ischemia-reperfusion injury.
Platelet receptors and signaling cascades the protect from inflammatory hemorrhage. Increase in inflammatory bleeding by inhibition of the main receptors GPIIBIIIA, GPIb, GPVI and CLEC-2 expressed on the surface of platelets. Although single inhibition of GPIIBIIIA in models of acute lung injury, LCMV infection, cerebral ischemia–reperfusion injury (IRI) and cremaster inflammation using direct receptor antibody or whole-body knockout were sufficient, combined blockage of GPIIBIIIA and GPVI aggravated the bleeding phenotype in the models of acute lung injury and peritonitis. GPIIBIIIA outside-in signaling is targeted through the Gα13/c-Src/14-3-3ζ complex or Arpc2, relevant in actin nucleation and lamellipodium formation. GPIIBIIIA and GPVI downstream pathways increase intracellular Ca2+ concentrations in a Syk- and PLCγ2-dependent manner, resulting in PS expression on the outer platelet membrane. Inhibition of CypD, as part of the mitochondrial permeability transition pore (mPTP) formation, or transmembrane protein 16F, a calcium-dependent scramblase, decreases PA and aggravates inflammatory bleeding. Local recruitment of coagulations factors to PS-positive platelets can be directly inhibited by anti-FIIa/-FXa, interfering with inflammatory hemostasis. GPIb can be inhibited by a whole-body knockout or blocking antibody of its ligand von Willebrand factor (vWF) in acute lung injury and dermal rpA. Inhibition of CLEC-2 or its downstream effector Src-homology leucocyte protein 76 are sufficient to enhance inflammatory bleeding in acute lung injury, while in dermal rpA simultaneously blockage of CLEC-2 or its ligand podoplanin and GPVI is necessary. Ablation of both alpha and dense platelet granules is required to affect inflammatory hemostasis, eg, in cerebral ischemia-reperfusion injury.
Platelet migration and repositioning toward microinjuries
Because immune cell extravasation is a key aspect disrupting vascular integrity, it is critical that platelets position themselves at sites of transmigration. Given the small size of individual platelets, the vast endothelial surface area and multiple sites of potential leukocyte transmigration, it has remained obscure how platelets effectively fulfill this task. Recently, we described a platelet function particularly observed under inflammatory conditions, the capability to exert autonomous migration,5,76 which critically relies on both the actomyosin cytoskeleton and activation of signaling cascades, including GPIIBIIIA-mediated, Gα13/c-Src/14-3-3ζ–dependent outside-in signaling.53,75,76 Indeed, platelet migration was shown to aid in immune responses, eg, through bundling of invading bacteria and subsequent presentation to leukocytes.76 We could show that in inflammation, platelets strategically reposition themselves at sites of neutrophil extravasation, particularly at endothelial cell junctions, by sensing density gradients of deposited fibrinogen.53 This process, termed haptotaxis, is required to reach sites of injury and plug the holes left behind by infiltrating neutrophils.53 Genetic ablation of platelet migration in Arp2-deficient mice as well as its pharmacological inhibition using inhibitors of the lamellipodial Gα13/c-Src/14-3-3ζ complex was shown to block repositioning in the inflamed vessel, resulting in exacerbated inflammatory bleeding in models of LPS-induced acute lung injury and peritonitis as well as inflammation of the cremasteric microcirculation.53,75 We note that whether platelet migration is required for inflammatory hemostasis in other bleeding models, eg, tumor-associated hemorrhage, remains to be investigated.
Platelet effector functions involved in plugging inflammatory microinjuries
Without forming a thrombus to seal off injuries, it is still not entirely clear how platelets maintain or recover vascular patency under these conditions. Although lesion size (macroscopic vessel injury vs microscopic leakage sites among endothelial cells) may influence the tendency to form clots, peripheral priming of circulating platelets as well as local alterations in receptor exposure by endothelial cells contribute to differences in behavorial phenotypes. One of the observed phenotypes is direct sealing of endothelial microleakages by single platelets. This phenomenon has first been described in electron microscopy studies that identified these cells seemingly plugging endothelial defects at sites of inflammation and concomitant leukocyte extravasation.77,78 Platelets, although small in size (diameter 1-2 μm in mice), can greatly enhance their surface area by recruiting membrane from their open canalicular system, with platelets undergoing shape change and forming lamellipodia in vitro averaging up to 10 μm2, which could help to seal off injuries. Recent work by Schurr et al, however, has called this into question. Using a mouse model deficient in platelet lamellipodium formation, the PF4cre-Cyfip1fl/fl mouse, the authors observed no increase in inflammatory bleeding in these mice.79
Another mechanism is platelet degranulation, which is essential for preserving vascular integrity in models of cerebral ischemia–reperfusion injury and in tumor-associated hemorrhage.39,44 Notably, only genetic ablation of both dense and α granule secretion was sufficient to aggravate inflammatory hemorrhage in the brain.44 In contrast, pulmonary and skin hemorrhage occur independently of this process.44 Platelet secretion is also critical in preventing intratumor hemorrhage.39
Braun et al recently provided mechanistic evidence that platelet secretion of angiopoietin-1 (Angpt1) after binding to vWF activated the endothelial receptor Tie2 at sites of leukocyte transmigration.80 Downstream of Tie2, the Cdc42 GTPase exchange factor FGD5 was crucial to prevent leakage of microspheres or fluorescently labeled dextrane as a proxy for inflammatory hemorrhage, likely through reinforcing cortical actin and junction stability among endothelial cells.81 Similar to the early landmark study by Goerge et al,32 leukocyte depletion prevented endothelial leakage in postcapillary cremasteric venules while blocking platelet-vWF interactions impaired endothelial integrity.80
Inflammatory hemostasis also requires the subsequent recruitment of plasmatic coagulation factors, because using clinically approved anticoagulants, such as enoxaparin, rivaroxaban, or argatroban that target FX and FII, respectively, aggravated pulmonary hemorrhage.68 Interestingly, in this model, fibrin recruitment to the pulmonary vasculature was dependent on platelets, because thrombocytopenia severely reduced fibrin formation and led to uncontrolled hemorrhage. In this context, we could show that PA of platelets links local platelet activation to the efficient recruitment of coagulation factors.68,82,83 PA was dependent on cooperative GPVI and GPIIBIIIA engagement, reflecting fibrin(ogen) and subendothelial collagen binding. This led to supramaximal Ca2+ bursts followed by the activation of cyclophilin D (CypD) and subsequent exposure of phosphatidylserine (PS) through the transmembrane scramblase transmembrane protein 16F (TMEM16F).46,68,84-86 PA in turn arrested migrating platelets at sites of collagen exposure and ensured local provision of PS+ negatively charged membranes. Binding of plasmatic coagulation factors targeted these to sites of microinjuries marked by collagen exposure, preventing uncontrolled intravascular clotting. We also provided evidence of platelet PA in LPS-induced peritonitis, in which procoagulant platelets were induced at sites of neutrophil extravasation and recruited plasmatic fibrinogen. Pharmacological blockade of platelet PA in this model aggravated inflammatory bleeding, suggesting a broader role for platelet hyperactivation in inflammatory hemostasis.68
Although these aspects—procoagulant function, potentially direct plugging, secretion, and endothelial cell signaling—play a role depending on context, it is important to note that deficiencies in these effector mechanisms do not fully recapitulate the severe phenotype of platelet depletion. The same holds true for the deletion of implicated receptors. Therefore, it is likely that these processes are redundant to some extent and that there might be additional, so far undescribed, effector and recruitment mechanisms in place. An overview of platelet effector functions and their impact on inflammatory hemostasis in common bleeding models is provided in Table 3.
Cellular platelet functions mediating inflammatory bleeding
| Functions and phenotypes . | Models with aggravated bleeding upon inhibition . | No bleeding observed upon inhibition . | Reference . |
|---|---|---|---|
| Migration | Acute lung injury, cremaster muscle inflammation | rpA | 53,68,87 |
| Degranulation/secretion | Cerebral IRI (tMCAO) in Unc13d−/−Nbeal2−/− DKO mice, tumor bleeding | Acute lung injury, rpA, cerebral IRI (single Unc13d−/− or single Nbeal2−/− KO mice) | 39,44,88,89 |
| PA | Acute lung injury, LPS-induced peritonitis | — | 68 |
| Plasmatic coagulation | Acute lung injury (anti-FIIa, anti-FXa) | — | 68 |
| Lamellipodium formation | — | Acute lung injury, rpA | 53,79 |
| Thrombocytopenia (<10%) | Acute lung injury, cremaster muscle inflammation, cerebral IRI [tMCAO], tumor bleeding, glomerulonephritis, rpA, increased skin permeability even in the absence of inflammation | Autoimmune arthritis | 31,32,35,36,39,42-44,49,50,53,65,67,68,79 |
| Functions and phenotypes . | Models with aggravated bleeding upon inhibition . | No bleeding observed upon inhibition . | Reference . |
|---|---|---|---|
| Migration | Acute lung injury, cremaster muscle inflammation | rpA | 53,68,87 |
| Degranulation/secretion | Cerebral IRI (tMCAO) in Unc13d−/−Nbeal2−/− DKO mice, tumor bleeding | Acute lung injury, rpA, cerebral IRI (single Unc13d−/− or single Nbeal2−/− KO mice) | 39,44,88,89 |
| PA | Acute lung injury, LPS-induced peritonitis | — | 68 |
| Plasmatic coagulation | Acute lung injury (anti-FIIa, anti-FXa) | — | 68 |
| Lamellipodium formation | — | Acute lung injury, rpA | 53,79 |
| Thrombocytopenia (<10%) | Acute lung injury, cremaster muscle inflammation, cerebral IRI [tMCAO], tumor bleeding, glomerulonephritis, rpA, increased skin permeability even in the absence of inflammation | Autoimmune arthritis | 31,32,35,36,39,42-44,49,50,53,65,67,68,79 |
Platelets in steady-state mucosal and skin vascular integrity: 2 sides of the same coin?
If acute thrombocytopenia per se, as observed in immune thrombocytopenia, causes overt bleeding by itself is still debated.90,91 New insights were provided by Gupta et al, who showed that severe thrombocytopenia does not cause erythrocyte extravasation but increases vascular permeability for small molecules (40 kDa), in line with previous studies showing ultrastructural alterations of the vasculature without disruption of the basement membrane after thrombocytopenia.72,92,93 In this context, genetic or pharmacological depletion of GPVI, its downstream effector phospholipase C gamma 2 as well as dense granule deficiency in both Unc13d−/− and BLOC1−/− knockout mice increased endothelial permeability, whereas neither protease-activated receptor 4, CLEC2 nor α-granule deficiency in Nbeal2−/− knockout had a measurable impact on vascular integrity.72
It is important to note, though, that severe thrombocytopenia (<20,000/μL in humans) strongly increases risk of bleeding in the absence of overt trauma, most likely if minor additional challenges arise, potentially including (minor) inflammatory reactions, mechanical stress, elevation in blood pressure, and preexisiting quiescient lesions.93,94 Interestingly, predilection sites include mucosal membranes, with epistaxis, gum bleeding, gastrointestinal (GI) bleeds, and hematuria representing the most frequent bleeding events in patinets with thrombocytopenia. Under these conditions, platelet-mediated hemostasis without clot formation is again the crucial element to maintain vascular integrity.91 Experimental data highlight that under these conditions, cross talk of platelets with endothelial cells, and release of both proangiogenic cytokines and growth factors are decisive to maintain endothelial junction integrity.91,95 A study investigating gastric mucosal bleeding showed that platelet aggregation is dispensable in this setting.96 Similar mechanisms seem to be of relevance in the skin microvasculature with petechiae forming in severe thrombocytopenia.97 In these settings, the exact contribution of platelet receptors, released factors, and effector functions including migration and procoagulant function are so far insufficiently understood.
Immunomodulatory and proresolving effects of blood leakage at sites of inflammation
Given that some endothelial leakage appears inevitable in certain settings, one may ask whether spillover of blood contents into the extravascular compartment is always detrimental or whether cells and plasma proteins may contribute to shaping the local microenvironment and, possibly, even immune responses.
Indeed, hemorrhage occurring in damaged tissues affects macrophage polarization toward an anti-inflammatory phenotype; hemoglobin originating from apoptotic and phagocytosed red blood cells was shown to promote anti-inflammatory programs in macrophages in models of brain hemorrhage, inflammatory colitis, and coronary artery plaque rupture.98-102 A recent study provided evidence that hemorrhage-associated heme accumulation in inflamed intestines induced CD163- and Spi-C–dependent transcriptional changes in resident macrophages, which led to reduced production of interleukin-6 and interleukin-1α, rendering mice resistant to colitis-associated organ damage.101,103 Interestingly, heme is also known to activate murine and human platelets via CLEC-2.62 Whether concomitant accumulation of blood-derived heme and platelets at the site of injury and subsequent, extravascular platelet activation through the CLEC-2/heme axis may also contribute to immunomodulation remains to be investigated.
In addition, localized and spatiotemporally restricted decreases in vascular patency may also provide damaged tissue with growth factors and immune cells that promote convalescence. Indeed, Wachaiyo et al showed that increased hemorrhage in CLEC-2- and GPVI-deficient mice promoted efficient wound repair through providing growth and coagulation factors locally.63 This was associated with a reduction in both proinflammatory cytokines, such as tumor necrosis factor α and cell types, such as neutrophils and M1-polarized macrophages, which fostered angiogenesis and increased wound repair compared to that seen in CLEC-2-/GPVI-proficient mice.
In summary, impaired integrity of the vascular barrier and subsequent inflammatory bleeding, if spatiotemporally limited, may have both beneficial effects on local reprogramming of the immune cell landscape and serve as a potential therapeutic target in both wound healing and cancer treatment.
Clinical applications for targeting inflammatory hemostasis pathways
Bleeding associated with inflammation is not only observed in mice but also contributes to morbidity and mortality in patients. GI bleeding as well as intracranial hemorrhage are associated with sepsis,104,105 and recent work has highlighted the role of platelets in maintaining vascular integrity in the inflamed gut.48 Corresponding to acute lung injury models, states of systemic inflammation, including autoimmune disease, such as vasculitis and both viral and bacterial pneumonia were shown to cause alveolar hemorrhage in patients, with histological evidence confirming increased neutrophil influx and concomitant extravasation of red blood cells.106 To date, there is no targeted treatment to boost inflammatory hemostasis, with current guidelines recommending optimizing plasmatic coagulation parameters and platelet transfusion in thrombocytopenia. In addition, inflammatory bleeding is observed in patients with thrombocytopenia, eg, after cytoreductive therapy or bone marrow transplantation.
Interestingly, nontraumatic bleeding is also observed as a side effect of antineoplastic therapy. In patients treated with dasatinib, a clinically approved dual c-Src and BCR/ABL inhibitor used as 1 of several treatment options in chronic myeloid leukemia or Philadelphia chromosome–positive acute lymphoid leukemia (ALL), a bleeding rate of up to 40% was reported in early clinical trials.107-112 This bleeding, which mainly accounted for mucosal or GI hemorrhage, was also observed in patients with nonthrombocytopenia.108,109 We have recently reported that c-Src orchestrates platelet migration. In line, platelets isolated from patients with chronic myeloid leukemia treated with dasatinib, who were in stable remission and not with thrombocytopenia, were unable to migrate on fibrinogen/ or albumin matrixes, whereas other platelet functions, at least partially, remained unaffected.75 Given the crucial role for migration in platelet repositioning under inflammatory conditions, our findings offer a possible explanation for observed bleeding phenotype in patients treated with dasatinib. We note that previous work shows impaired platelet effector functions in patients who are chronically treated with Src inhibitors,108,109,113,114 and that inhibition of these effector functions, including platelet aggregation and PA, may also contribute to the clinical phenotypes observed in these patients.
Targeting inflammatory hemostasis through inhibition could also serve as an intriguing therapeutic opportunity. Thrombocytopenia-induced increases in permeability of tumor vessels were shown to selectively enhance local accumulation of antineoplastic therapeutics and promote tumor cell death.35,70 Furthermore, antibody-mediated or genetic depletion of GPVI exacerbated hemorrhage in 2 independent tumor models, which in itself decreased proliferation, increased cancer cell death, and provided the basis for enhanced delivery of chemotherapy.67 This effect was dependent on neutrophil extravasation and could be mimicked through IV application of a soluble dimeric GPVI-Fc fusion protein, providing further evidence for clinical applications.67
Before such therapy becomes available, further studies will be necessary to clarify putative off-target effects, such as enhancing vascular access for invading and metastatic malignomas, and hemorrhages at unwanted sites, such as the lung or the brain. In addition, whether inflammatory hemostasis shares pathophysiological features in mice and patients requires future investigation, eg, whether therapeutic or hereditary GPVI deficiency, as observed in some Chilean families, may also affect inflammatory hemostasis.115-117
We note that almost all of the above-mentioned platelet receptors and pathways are not only solely used when platelets combat inflammation-associated vascular leakage but also play pivotal roles in shaping the inflammatory and/or thrombotic response in the first place. Regarding shaping the inflammatory and/or thrombotic response, Pircher et al described ITAM-mediated platelet activation through GPVI and downstream c-Src/Syk to critically contribute to arterial thrombosis through promoting neutrophil extracellular traps formation.118,119 In addition, these signaling pathways may serve as therapeutic targets in the context of “thromboinflammation,” ie, activation of platelets and coagulation factors through soluble and cellular components of the immune system after a primarily inflammatory stimulus, without subsequent occlusive thrombus formation (reviewed elsewhere120,121). Indeed, according to this definition, recruitment of inflammation-primed platelets to endothelial leakage sites induced by neutrophils may be regarded as thromboinflammatory sequelae. Furthermore, it should be emphasized that the involvement of multiple platelet glycoproteins known to affect hemostasis has thus far not been investigated in the context of inflammatory bleeding. Given exciting novel insights into platelet-coagulation interplay, such as the recently described role of GPV in spatiotemporally governing fibrin formation,122 systematic investigation using inflammatory bleeding models will aid to broaden our understanding of inflammatory hemostasis.
Several other aspects will need to be addressed in the experimental setting, such as, why is inflammatory bleeding trigger-dependent? How do different vascular beds, specifically endothelial cells, affect the dependency on platelet receptors and signaling cascades as to whether bleeding occurs or not? Can we attribute some of these differences and dependencies to leukocyte factors, such as circadian rhythmicity or intrinsic heterogeneity?123-125
Answering these questions within the scope of translational research is likely to reveal novel therapeutic gateways. These answers may also inform us as to whether modern therapeutic agents, including recombinant coagulation factors, polyvalent humanized antibodies, polymer-based hemostatic agents, or even platelet-mimicking nanoparticles may be usable to rescue inflammatory bleeding.117,126-131
Conclusion
Pivotal studies published during the past decade have shed further light on the complex pathophysiology that underlies hemostasis without clot formation. Their findings provide ample evidence that stopping inflammation-associated hemorrhage requires activation of distinct platelet signaling hubs and, importantly, a behavioral platelet phenotype that drastically differs from thrombus formation and classical, trauma-induced hemostasis. Importantly, and in contrast to both classical hemostasis and thrombosis, platelets predominantly act as single cells rather than syncytial clots, which allows for unprecedented versatility, migration-mediated mobility, and swift reactions to the changing microenvironment. As a more nuanced picture of platelet function emerges (a dichotomous conundrum of the activated, aggregating platelet in thrombosis, and classical hemostasis vs an equally active, but more diversely reacting platelet during inflammatory processes) the exciting field of inflammatory hemostasis advances. We have gained more insights into the factors that govern many of its mechanisms, but open questions regarding the dependency on the inflammatory stimulus and on organ-specific vascular beds will have to be addressed before therapeutic leverage can be reached.
Acknowledgments
This study was supported by Deutsche Forschungsgemeinschaft, Clinician Scientist Program PRIME 413635475 (R.K.), research grant 512460086 (L.N.), SFB1123 Project B06 (L.N.), Deutsche Herzstiftung e.V., Frankfurt a.M. (R.K.), and the German Centre for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislauf-Forschung e.V. [DZHK]), Clinician Scientist Programme (L.N.), and Shared Expertise Grant (R.K., L.N.). This study was also supported by the Else Kröner-Fresenius-Stiftung (R.K.), the Friedrich-Baur-Stiftung (R.K.), the LMUexcellent programme (R.K.), the Grimmke-Stiftung (L.N.), and the German Society for Thrombosis and Hemostasis (L.N.). All figures were created using BioRender.com.
Authorship
Contribution: R.K., R.E., and L.N. reviewed the literature and conceptualized the manuscript; R.K. wrote the initial draft; R.E. prepared the figures; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Rainer Kaiser, Medizinische Klinik und Poliklinik I, University Hospital Ludwig Maximilian University Munich, Marchioninistr 15, 81377 Munich, Germany; e-mail: rainer.kaiser@med.uni-muenchen.de; and Leo Nicolai, Medizinische Klinik und Poliklinik I, University Hospital Ludwig Maximilian University Munich, Marchioninistr 15, 81377 Munich, Germany; e-mail: leo.nicolai@med.uni-muenchen.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal